Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Review Article - Open Access, Volume 6
Clinical application value of imaging-AI in bronchopulmonary dysplasia of prematurity
Shuzhe Xiao1#; Lewen Zhou2#; Lingling Wang2; Qi Zheng2; Jie Yang1*
1Department of Neonatology, Nanfang Hospital, Southern Medical University, China.
2The First Clinical Medical College of Southern Medical University, China.
#These authors have been equally contributed to this article.
*Corresponding Author : Jie Yang
Department of Neonatology, Nanfang Hospital,
Southern Medical University, Guangzhou, 510515,
China.
Email: Jieyang0830@126.com
Received : Jan 11, 2025
Accepted : Feb 31, 2025
Published : Feb 07, 2025
Archived : www.jcimcr.org
Copyright : © Yang J (2025).
Abstract
Bronchopulmonary Dysplasia (BPD) is a prevalent respiratory disorder among premature infants, contributing to heightened infant mortality rates and imposing significant social and economic burdens on families. The long-term impacts of its prognostic complications have been well-documented. Therefore, timely diagnosis and intervention are crucial for the progression and outcomes of BPD. Examining clinical methods for diagnosing BPD assumes paramount importance. In this review, we explore various imaging examinations that assist in diagnosing BPD, grounded in its pathophysiology, augmented by AI-assisted analysis. We delve into their clinical application values, and assess their strengths and limitations in clinical diagnosis and treatment, thereby aiding clinicians in providing a comprehensive, precise, and expeditious diagnostic and therapeutic approach for BPD in premature infants.
Keywords: BPD; Imaging; AI.
Citation: Xiao S, Zhou L, Wang L, Zheng Q, Yang J. Clinical application value of imaging-AI in bronchopulmonary dysplasia of prematurity. J Clin Images Med Case Rep. 2025; 6(2): 3459.
Introduction
BPD characterized by impaired alveolar formation and abnormal vascular development is still associated with increased mortality and morbidity [1,2]. After introducing lung protective ventilation strategies, BPD represents a developmental arrest of the preterm lung in the saccular and alveolar stages of lung development. Disruption of pulmonary septation, alveolarization, and vascularization in this critical window results in a lung with fewer, but larger and dysfunctional alveoli [3]. Currently, clinical research hotspots in this field mainly focus on the genetic factors of BPD in preterm infants, mechanisms of lung hyperoxia and inflammation, early intervention strategies, and precise diagnosis and treatment. The application of imaging in preterm infants is a necessary aspect to assist clinical practices in achieving accurate diagnosis and treatment of BPD. Since there is currently no specific or effective available treatment for this entity, there is a need to assess early the premature newborns with the highest risk of the disease to test suitable therapies, as well as to evaluate the evolution of the disease after admission, using different imaging exams [4]. Therefore, it is necessary to formulate a reasonable diagnostic plan for BPD to determine the occurrence and severity of the disease, which facilitates rapid, timely and accurate treatment and medication and provides respiratory support. At the same time, the development of modern science has made artificial intelligence-assisted medicine possible. This article reviews Chest X-Rays (CXR) and lungs. Articles on Ultrasound (LUS), Computer Tomography (CT), and Magnetic Resonance Imaging (MRI) analyze the auxiliary diagnosis of AI in various imaging technologies and introduce the application of imaging combined with AI in the diagnosis of BPD.
Etiology, pathological lung and pathogenesis of BPD
Etiology and pathogenesis of BPD
Following the canalicular or saccular phases, postnatal lung development occurs in an oxygen-rich environment, which contrasts with the fetal pulmonary circulation. Consequently, the degree of prematurity is the most significant risk factor for the development of BPD. Postnatal lung injuries, aberrant remodeling in response to these injuries, and disrupted prenatal lung development all contribute to the pathogenesis of BPD [5]. Resuscitation at birth can start the injury that leads to BPD, and postnatal exposures (high oxygen exposure, mechanical ventilation linked to barotrauma, volutrauma, and infection) can exacerbate it. The injury that causes BPD probably starts as altered lung development before delivery in many infants (small for gestational age, chorioamnionitis, tobacco exposure) [6]. Maternal factors, genetic predisposition, ventilator-associated lung injury, oxygen toxicity, sepsis, Patent Ductus Arteriosus (PDA), and dietary inadequacies are some of the prenatal and postnatal factors that have an impact on its development [7].
Pathological lungs and normal lungs
Table 1: Difference between pathological lung and normal lung.
| Normal lungs | Pathological lungs | |
|---|---|---|
| Lung structures | Mostly smooth, moist, and reddish, the lung’s microvasculature matures and its alveoli multiply during pregnancy and the postnatal period [8,9] | Disrupted alveolarization and microvascular development lead to aberrant lung mechanics and gas exchange [9] |
| Image | On a chest X-ray film, the normally aerated lungs appear as uniformly translucent areas known as lung fields, exhibiting comparable translucency bilaterally. The hilar regions of the lungs display branching opacities that resemble a tree-like pattern radiating outward | Alveolarization, fibrosis, cystic emphysema, and some changes in the airways, such as trachea and bronchomalacia, as well as subglottic stenosis [10] (will be subdivided in the future) |
| Stage | [5], embryonic; pseudoglandular; canalicular; saccular; alveolar [11] | Assuming a viability limit at 24 weeks PMA, the majority of preterm babies are in the saccular and canalicular phases of lung morphogenesis [12] |
| Pathophysiology | / | Necrotizing bronchiolitis, large airway injury, pulmonary arterial wall thickening, diffuse emphysema, pulmonary fibrosis, and cystic changes in the lung parenchyma [13] |
X-ray (Chest X ray) in BPD
Historically, CXR has been the preferred imaging modality for BPD due to its ease of bedside administration, ability to detect acute changes, and effectiveness in screening for significant abnormalities such as consolidation or pneumothoraces. However, guidelines now recommend reserving outpatient imaging involving ionizing radiation for specific subgroups of infants and children with BPD, particularly those exhibiting severe symptoms or experiencing recurrent illness [14].
Texture thickening, strip or speckle shadow, texture disorder, high transparency, and other desirable characteristic changes can be seen in CXR pictures as the BPD develops. Premature newborns with BPD may have increased lung density without typical lung markings on CXR [15]. Radiographic confirmation of parenchymal lung disease by CXR has been incorporated into a definition of BPD to establish a severity grading system that allows the identification of patients at risk of poor respiratory outcomes [16]. In preterm infants with BPD, a lower chest area on CRTA compared to the normal range indicates the need for CXR evaluation [17]. X-ray findings in the diagnosis of neonatal BPD typically include: lung hyperinflation, linear hyperdensities, and subpleural triangular opacities [18].
Northway et al. separated the results of the chest X-ray into four stages based on the pathological course of BPD. In Stage I, the NRDS appears as blurred ground glass opacity in both lung areas, which is the same as in Stage II. Completely opaque and white lung-like, both lungs are in Stage III, which marks the beginning of chronic pulmonary fibrosis. In Stage IV, the density of the two lung fields is unequal, and linear or patchy shadows are seen along with inflated translucent tiny sac spaces. The two lung fields’ transparent regions are vesicular and expanded, exhibiting structural abnormalities, hyperinflation, atelectasis, patchy opacities, and dispersed strips [19].
Currently, chest images are taken by placing the X-ray box on the back of the child’s chest while lying on the child’s back. However, because X-rays show tissue anatomy based on differences in tissue absorption, their strong penetrating ability is susceptible to radiation injury to children during use in neonates. In addition, x-rays are inaccurate in describing lung ventilation and the overall severity of parenchymal disease due to their flat anteroposterior view, and the combination of chest CT, LUS, and MRI makes up for the shortcomings of X-rays.
LUS in BPD
LUS is a rapid, reproducible, radiation-free, point-of-care method that is simple to learn. Age has little bearing on LUS symptoms [20], which makes it particularly appropriate for usage in critical care settings and with the tiniest patients [21].
LUS shows vertical lung artifacts in normal physiological lungs. The pleural line, a horizontal, high-echogenic, uniform line with regular and orderly artifact spacing, is what defines the A-line. There are also apparent lung sliding and lung pulse, as well as short vertical artifacts that originate from the pleural line and follow its movement (formerly known as Z-lines). The physiological lung pattern (A pattern) is what this pattern is known as [22,23]. In contrast, dense lung B-lines with strong echogenicity and a comparatively high number of artifacts are seen in LUS findings in the context of BPD. The A-lines are blurred, and the pleural line appears thick and uneven with lung consolidations, pulmonary edema, and subpleural tiny consolidations. Additionally, the alveoli exhibit bilateral diffuse and irregular interstitial patterns with consolidation of the broncho- gram. A pulsing indication could be a symptom of atelectasis [24].
Establishing LUS program in the Neonatal Intensive Care Unit (NICU) reduces the number of chest X-rays by around 50% and considerably lowers the average radiation dose that patients obtain, according to research by Guillaume Escourrou et al [25]. Further, considering LUS is essentially distinct from chest X-rays in that it measures the amount of lung fluid, whereas X- rays evaluate lung ventilation directly, its appearance remains unchanged following surfactant transmission [26]. Lung ultrasonography can also detect the severity of BPD by monitoring the patient’s hemodynamic changes. It determines the severity of right ventricular overload by detecting tricuspid regurgitation, dilation of the right atrium and right ventricle, and dilation of the inferior vena cava. This information helps to determine the patient’s pulmonary artery blood oxygen saturation and the presence of pulmonary vascular injury, allowing an assessment of changes in the patient’s condition and severity [27].
CT in BPD
One kind of Computed Tomography (CT) imaging technique for assessing cross-sectional imaging is CT. High-resolution CT offers substantial clinical application value since it can diagnose BPD at an early stage and has a density resolution that is much higher than X-rays. It also makes it easier to image intricate anatomical components of the lungs.
Clinical practice for BPD utilizes CT for imaging and scoring, including architectural distortion, hypo-attenuated and hyper-attenuated areas, and subpleural opacities. Three new Hounsfield Units (HU)-based scoring methods, the quantitative scoring system constructed by SPIEBERG et al. and the manual PRAGMA-BPD scoring method [28-30]. Under a CT microscope, the pulmonary blockage brought on by alveolar septal fibrosis, coarse reticular lung turbidity, and lung parenchymal hyperdilation can be observed. Reduced perfusion, thickening of the bronchial wall, decreased ratio of bronchi to pulmonary artery diameter, linear opacity, bulla, localized low attenuation, multifocal attenuation, and extensive bilateral lung density [31], which exhibits peribronchial enhancement, septal thickening, emphysema with tiny cysts, linear and nodular opacities, and pulmonary mosaic-like density [32].
The diagnostic process for BPD in neonates relies heavily on chest CT. However, because its usage necessitates radiation exposure and patient sedation, it is typically not the first option in clinical practice. The usage of sedatives and radiation dosages in CT has declined as imaging technology has advanced. Additionally, CT is now used more often to examine patients with mild to moderate BPD due to its advantages over X-rays in terms of higher resolution and clearer early imaging. In contrast, alternative imaging technologies are also worth implementing in clinical settings.
MRI in BPD
Since CT has limits in longitudinal assessment and radiation hazards, MRI has emerged as a crucial technique for assessing bronchopulmonary structure and function [33,34]. MRI is capable of providing significant image-based measurements of lung morphology that correlate with the severity of BPD and clinical outcomes without requiring anesthesia or sedation [35]. Proton MRI, oxygen-enhanced MRI, and ultra-short echo time MRI are examples of MRI techniques.
In order to counteract the rapid signal decay in the lung parenchyma, proton MRI employs signal averaging techniques and short echo periods. Without changing the hardware of the scanner, oxygen-enhanced MRI makes use of the physical characteristics of molecular oxygen. Extremely brief TEs are used in ultra-short echo time MRI to enhance the signal from the lung parenchyma.
MRI can detect and evaluate trustworthy imaging markers of BPD in lung applications. According to studies, a higher overall risk of BPD is linked to both longer lung T₂ relaxation times and shorter T₁ relaxation times. While shorter T₁ relaxation times may signify emphysematous alterations or relative changes in lung perfusion, longer T₂ relaxation times may indicate greater interstitial remodeling [36].
However, MRI is complicated and has only played a limited role on account of technical issues. In addition to aberrations from cardiac and respiratory motor sources that further deteriorate image quality, the low ¹H density of the lung parenchyma (only 20% of solid tissue) results in numerous air-tissue interfaces with rapid signal attenuation (i.e., short T₂*, 1.5<1.2 ms at 1.5 T). MRI of the lungs can be enhanced through a variety of methods since the magnetic characteristics of the lung cause the MRI signal of the lung parenchyma to attenuate considerably more quickly than that of other tissues [37].
AI and image
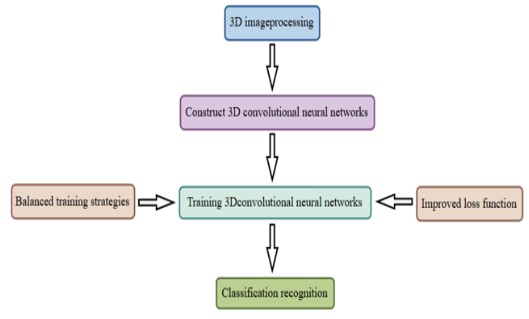
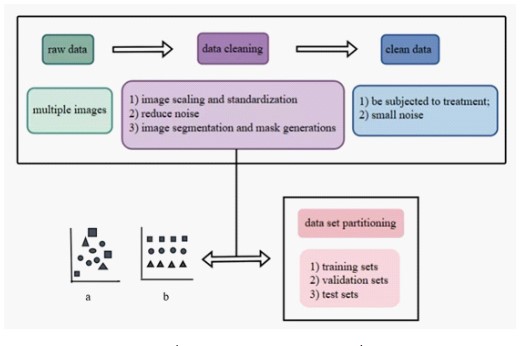
Machine Learning (ML), Deep Learning (DL), and Reinforcement Learning (RL) are the three main categories into which Artificial Intelligence (AI) can be separated at the algorithm level. From raw data, such ultrasound scans, DL can directly process and collect intermediate and advanced features. Then, using the learnt features, it can make wise conclusions. By treating images as numerical sequences without cognitive bias or the requirement for spatial pixel connections, DL can evaluate quantitative patterns that may reveal insights beyond human interpre- tation, strengthening human diagnostic capabilities [38]. It has been demonstrated that DL models can execute tasks including image classification, localization, object detection, and segmentation on rate with or even better than humans [39,40]. Data collection is a precondition for deep learning. Before being used for model training, the gathered data will undergo procedures including data cleaning (removing inaccurate, duplicate, etc.) and data annotation. Neural network topologies including Convolutional Neural Networks (CNN), activation functions, optimization methods, evaluation, and more are all included in model training.
AI-DL
Training datasets [41]:
Table 2: Different training datasets sets.
| Constitution | Measure | Evaluation criteria | |
|---|---|---|---|
| Lung segmentation dataset | 1491 images: 1403 chest radiographs from the preterm infant cohort that were easy to label and 88 chest radiographs from the term infant cohort | Mark the lung area: The lung fields are along the inner side of the chest wall and the margin of the mediastinum. | Dice index |
| BPD prediction dataset | 1021 images taken at ≤168 h postnatal age | Further divided into two subgroups of ≤24 h postnatal with 505 images and 25–168 h postnatal with 516 images after delivery to analyze the performance of the BPD prediction AI model in these two different periods (alleviate the treatment effect from surfactant) | Sensitivity, specificity, precision, accuracy, and F1-score |
Data pre-processing
Data pretreatment for X-ray-based BPD prediction usually includes feature extraction, data cleaning, machine learning model training, optimization, and image scoring systems in addition to data collection and management. In particular, birth weight, gestational age, and the length of oxygen therapy are among the clinical information and pictures of premature babies that are gathered. Chest X-ray films are evaluated using current X- ray scoring methods, such as those used for BPD staging and Respiratory Distress Syndrome (RDS) grading. Essential characteristics, such as lung transparency and the severity of emphysema, are then taken out of the X-ray pictures for additional examination. To guarantee the correctness and dependability of the data, abnormal or low-quality image data are eliminated from the gathered photographs. Afterward, moving on to the crucial stage of data preprocessing, Josh Williams et al investigated the automatic segment segmentation of lungs using clinical data and deep learning technologies (such convolutional neural networks) for imaging data analysis [42]. Furthermore, performance evaluation (including AUC, sensitivity, and specificity) and cross-validation guarantee the model’s generalizability across various data sets.
Model training and optimization
Based on the key image features extracted from diverse imaging modalities (such as X-ray, MRI, LUS, CT, etc., contingent upon specific research requirements) and the associated comprehensive clinical data, the machine learning model is trained. For the model architecture, a Multilayer Perceptron (MLP) [43] can be utilized due to its simplicity and versatility, which effectively addresses linearly separable problems. Alternatively, a self-attention mechanism network may be chosen for its proficiency in capturing long-range dependencies within the data and excelling in establishing associations between complex images and clinical information [44]. During the training process, both image features and clinical data are fed into the model, with appropriate initial parameter values set. A substantial dataset of known case samples enables the model to learn disease patterns and feature correlations, such as understanding the relationship between the chest X-ray image features of premature infants, along with clinical parameters like birth weight and gestational age, and the incidence of BPD.
A two-stage process was used to optimize the model’s performance. Predicting the duration of respiratory support (DSd) was the main goal in the first phase. Afterwards, in the second phase, efforts were focused on forecasting both the incidence and severity of BPD [45].
Model prediction and evaluation combined with clinical variables
To deliver accurate predictions for unforeseen circumstances, apply the thoroughly trained and refined model. The algorithm quickly produces prediction findings on the likelihood, severity, and duration of respiratory support of BPD after receiving the imaging characteristics and clinical information of new patients. Thoroughly assess the model’s performance indicators, particularly its AUC value, sensitivity, and specificity. These indicators can give clinicians valuable guidelines for making decisions [46].
AI and image
AI and Xray
A thorough literature review of AI-based CXR analysis tasks, including enhancement, segmentation, detection, classification, image and report generation, and various models for detecting related diseases, is presented by Yasmeena Akhter et al. [47]. Using deep learning algorithms, image preprocessing, and a summary of the datasets and metrics used in the literature, AI can automatically identify key imaging features of BPD from chest X-rays, such as pulmonary emphysema and changes in lung texture. This automated analysis not only speeds up the diagnostic process but also lowers human error, improving the accuracy of clinical decision-making.
With an emphasis on pneumonia analysis utilizing chest X-ray pictures, Syed Taimoor Hussain Shah et al. explores a variety of datasets and machine learning algorithms used in recent research for lung illness categorization. They investigate custom Convolutional Neural Networks (CNNs), ensemble approaches, pre-trained deep learning models, and traditional machine learning techniques. Data collection, preprocessing, feature extraction, and categorization utilizing machine vision, machine and deep learning, and Explainable Artificial Intelligence (XAI) are all covered in this thorough comparison of several classification techniques [48].
AI and LUS
The presence of artifacts, such as acoustic shadows, speckle noise, motion blurring, and missing boundaries, which are created as a result of the intricate interaction between US waves and mother and fetal biological tissues, can make fetal US image analysis difficult from the clinician’s point of view [49]. Further challenges include fast fetus movements, occluded anatomical structures (e.g., due to fetus positioning) and high variability associated with different gestational weeks.
LUS AI is primarily focused on prenatal and cardiac ultrasound and is limited in its ability to use and validate developed algorithms in clinical settings [50-52]. DL addressed investigated tasks like standard plane detection, biometry parameter estimation and anatomical structure analysis in a short time [49], which can be trained in a relatively short amount of time using large amounts of data, with similar or better performance compared with human operators.
AI and CT
There are restrictions in scientifically identifying patterns and assessing the amount of emphysema and ILD, despite the fact that chest CT scan subjectively diagnose these conditions, classify patterns, and assess their severity. To date, visual methods (visual evaluation and quantification) have been used to determine the type or amount of emphysema or ILD on chest CT scans. Although this method has been tried and found to be effective, it is still subjective and time-consuming. Consequently, AI-CT greatly aids clinical work [53].
In order to automatically and objectively identify patterns and the severity of pulmonary emphysema or interstitial lung disorders on chest CT scans, researchers have created a number of algorithms using Artificial Intelligence (AI). According to studies, a correlation between a rise in the relative percentage of emphysema and a reduction in lung function—one of the consequences of borderline personality disorder-is revealed by AI-based quantification of emphysema on chest CT images [54].
AI and MRI
Initial studies targeted lung volume measurements in MRI [55], whereas only a few studies explored the assessments of structural changes in the neonatal lung [56].
By using convolutional neural networks, Benedikt Mairhörmann and associates enhanced the resilience and application of DL techniques for MRI lung segmentation in preterm newborns. They assessed these structural traits’ added usefulness in categorizing infants based on the clinical diagnosis of BPD. By calculating Three-Dimensional (3D) MRI lung characteristics, mea- suring lung volume and shape, pixel intensity distribution, and lung surface, the DL lung segmentation ME shown outstanding segmentation performance [57].
Unfortunately, the 3D AI MRI lung features alone did not out- perform patient and clinical features. Supplementary analysis that ranked all features revealed that clinical measurements, such as gestational age, outperformed the MRI lung features [58].
AI application
In fact, AI algorithms enhance preinterpretive processes, including image reconstruction, image acquisition, and mitigation of image noise [59], playing a role in recognizing automatically, processing complex data and interpreting images for imaging technologies such as X-rays, lung ultrasound, CT, and MRI. However, AI training and the advancement of deep learning are time-consuming processes that require significant effort, cost, and engineering investment. Issues such as how to precisely standardize image data for AI training and how to ensure the accuracy and independence of AI in image processing are all considerations we must take into account when deploying AI.
There are advantages and disadvantages. AI has indeed brought unprecedented convenience to imaging applications, but when it comes to diagnostic results and the underlying ethical considerations, do AI systems adhere to established guidelines?.
Technical challenges
Although current clinical research can apply deep learning models to chest X-rays and so on and use AI to interpret imaging images of many lung diseases intuitively, this process requires support from large-scale data samples, and the non-standard nature of the images (e.g., biases in image acquisition, equipment, and image settings) may exacerbate the challenges of AI clinical application [60].
Ethical and legal aspects
Because of the uncertainty generated by the lack of scrutiny of the recommendations provided by AI algorithms, clinicians will be unable to take appropriate steps to mitigate their concern that algorithm inaccuracy could lead to patient injury and medical liability [61-63].
The revolution in AI that has shifted the governance relationship from a doctor-patient relationship to a doctor-machine-patient model has complicated the attribution of responsibility for medical malpractice litigation experts. Who should bear the responsibility for product liability and negligence liability is something to consider in ethics and law [63,64].
Application
Machine learning model
Dan Dai et al. performed a gene burden test to find risk genes with loss-of-function mutations or missense mutations over-represented in BPD patients. We then developed two predictive models for the risk of BPD by integrating patient clinical and genetic features. The performance of the models was evaluated using the Area Under the Receiver Operating Characteristic curve (AUROC) [65]. Combined BPD risk gene sets with basic clinical risk factors can thus accurately stratify BPD risk in premature infants.
Intelligent auxiliary software
If patients with BPD are in severe condition and complicated with cardiac insufficiency, continuous hypoxia and other complex situations, it may indirectly have a certain impact on the image quality during the coronary CTA examination or the patient’s tolerance. Deep Vessel-Analysis independently developed by Coreline Medical is a coronary intelligent auxiliary diagnosis system. It utilizes artificial intelligence technology to analyze coronary CTA and can fully automatically complete functions such as coronary artery reconstruction, three-dimensional visualization, and lesion detection, and generate structured reports. Clinical doctors can utilize this system to delineate the lesions, thereby making judgments about BPD.
Table 3: Comparison of imaging technologies and connection with AI.
| X-ray | LUS | CT | MRI | |
|---|---|---|---|---|
| Advantages |
① Good contrast, clear imaging, clear visualization of subtle lesions or thick parts, and objective records for review and comparison consultation and discussion ② Easy to review, low price, easy to inspect |
① Simple and fast operation can be done at the bedside ② No radiation |
① Multi angle and all-round inspection ② Extensive scan, multiplanar and reconstructed, clearly revealing the more insidious lesions, as well as the tissue involvement and the extent and extent of the lesions |
① It is a radiation-free alternative to CT, which has greater soft tissue contrast, dynamic study of respiratory mechanics, perfusion and ventilation imaging than CT ② Reduce radiation-related adverse reactions |
| Limitations |
① The operation is complicated, and it is not convenient to observe the activity and function of organs ② Radiation ③ 2D level ④ It is relatively insensitive to structural changes in the lungs of BPD ⑤ It is easy to be disturbed by clothing and personal belongings |
① Weak penetration ② LUS is related to technical proficiency and equipment supply |
Radiation |
① The patient’s small size, low spatial resolution, and the challenge of being sensitive to infant movement lead to blurring, ghosting, and other image artifacts ② Proton signal attenuation is fast; low proton density of the lungs and artifacts caused by high magnetic susceptibility and respiratory movements ③ MRI studies are affected by involuntary (breathing, cardiac, or peristalsis) movements ④ Those who have metal objects in their bodies, those who wear pacemakers, and those who suffer from claustrophobia should not be examined by MRI |
| AI Connection | Improve accuracy, automatic identification helps doctors test and diagnose with confidence | |||
Lung 3D drainage map model
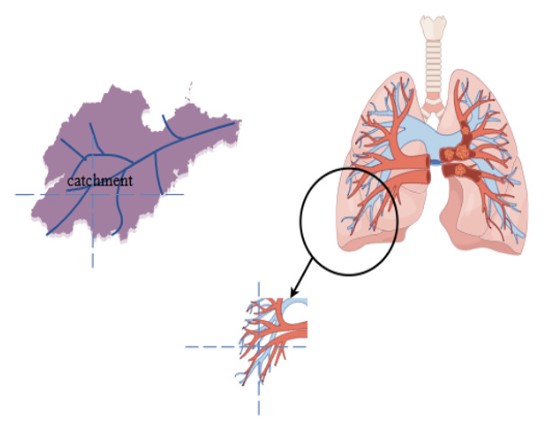
A sophisticated computer-aided technique for the accurate diagnosis and efficient treatment of lung illness is watershed analysis. Image imaging was used to display the lesion design, three-dimensional reconstruction was used to visually represent the relationship between the lesions and pulmonary blood vessels, and a customized pulmonary blood vessel network was created to create the boundary. These are AI surgery’s corner-stones. Physicians can more easily identify lung tissue with the use of the lung watershed map, minimizing mechanical injury and lung tissue removal during surgical intervention. This lowers the risk of intraoperative hemorrhage and cuts down on surgical time.
Through the combined identification of artificial intelligence algorithms and medical staff analysis, the use of lung basin maps efficiently prevents errors and shortcomings in the subjective identification of blood arteries in lung tissue and enhances surgical efficiency. BPD is a chronic pulmonary disease associated with prematurity. Its pathological mechanisms involve the pulmonary blood vessels and alveoli, including alveolar simplification, reduced secondary septation, and markedly abnormal microvascular growth, which lead to vascular remodeling, Pulmonary Hypertension (PH), and persistent pulmonary vascular diseases [66]. With the assistance of advanced imaging technologies and three-dimensional reconstruction algorithms, Zhongnan Hospital of Wuhan University developed a lung 3D drainage map model. This model visually represents the structural and functional characteristics of the lungs. While a direct relationship between BPD and the lung 3D basin map has not been explicitly established, there may exist a pathophysiological link between them. It is plausible that the image model could be utilized in the clinical monitoring of BPD shortly, thereby assisting clinicians in diagnosis and treatment.
Monitoring pulmonary hemodynamics and flow analysis, re-positioning and defining lung tissue margins and vascular network clusters, creating a lung watershed map unique to each preterm infant, and combining multiple imaging technology videos to determine the direction of surgery for zoning are innovative approaches that can be taken into consideration in future clinical work to investigate the primary focus of BPD in preterm infants. By using precise imaging analysis and customized treat- ment plans, this method seeks to enhance the prognosis of BPD patients and lower the prevalence of chronic lung illness and long-term hospitalization.
The region influenced by rivers on soil closely mirrors the area served by pulmonary vessels to lung tissue. The estuary, serving as the primary outflow channel, can be compared to the hilum of the lung. The geographical boundary of a basin, representing planar structure, parallels the intersegmental planes of lung functional units. Furthermore, the morphological characteristics of rivers in hydrological processes can be likened to the distribution of arteries and veins within the lung.
Future and challenges
With the further development and deepening of the discussion of BPD in modern medicine, various technologies have served the clinical cause more comprehensively and progressively, and X-ray, CT, MRI and LUS have been widely used in clinical practice, providing accurate judgment and visual data sup- port for patient diagnosis and disease prediction, and turning qualitative into quantitative.
Compared with other methods, X-ray imaging is fast, inexpensive, and widely used in clinical practice, but there are also limitations that the sensitivity is lower than that of other imaging techniques and there are few indications, so that the clinical detection of BPD is not comprehensive. Compared with the above techniques, CT imaging has high resolution and sensitivity, and can provide cross-sectional images without tissue overlap, but due to its radiation effect, CT is not widely used in the diagnosis and treatment of neonatal BPD. LUS is used in the emergency treatment of BPD because of its simple and convenient operation and bedside operation, but as an imaging method with weak penetrating ability, it also has shortcomings in predicting the deep structure of the lungs. MRI can be regarded as an alternative to CT imaging without ionizing radiation, which can be used for the diagnosis and treatment of BPD with multiple sequences and directions. However, it is not as effective as imaging other organs and tissues in the lungs, which lack protons, and is prone to artifacts.
Therefore, when to use what imaging method is more important to consider in clinical practice. The flexibility and speed of X-ray make it play a significant role in the initial diagnosis and short-term diagnosis; CT is a restrictive force for X-rays due to its high definition and may be considered when X-ray results are uncertain or when further analysis of lung structures is done. In clinical practice, the frequency of use of LUS and MRI is not as high as the first two, but LUS has a positive diagnostic effect in assessing cardiac function and hemodynamic changes, as well as detecting complications such as pulmonary hypertension. Due to its ability to image soft tissues with high resolution, MRI can be used to assess the subtle structure and degree of lesions in the lungs and can be used as a final intervention for imaging diagnosis when other techniques cannot meet the clinical diagnosis.
In the future, a series of combined applications of imaging and artificial intelligence will be both an opportunity and a challenge for modern medicine, which requires the joint efforts of AI designers and clinical imaging doctors to use big data and gold standards for AI training and diagnostic review. AI imaging will also unswervingly assist clinical work in diagnosis, treatment and prevention, specify personalized imaging examination methods according to the specific conditions and clinical needs of children, clarify the advantages and disadvantages, applica- bility and limitations of each imaging technology, and cooperate with artificial intelligence to improve clinical technology better and more conveniently.
Declarations
Ethical approval: This declaration is “not applicable”.
Funding: This work was supported by National Natural Science Foundation of China (82171714).
References
- Tenero L, Piazza M, Sandri M, Ferrante G, Giacomello E, et al. Early Diagnosis of Bronchopulmonary Dysplasia with E-Nose: A Pilot Study in Preterm Infants. Sensors (Basel). 2024; 24: 6282.
- Dankhara N, Holla I, Ramarao S, Kalikkot Thekkeveedu R. Bronchopulmonary Dysplasia: Pathogenesis and Pathophysiology. J Clin Med. 2023; 12: 4207.
- Wozniak PS, Makhoul L, Botros MM. Bronchopulmonary dysplasia in adults: Exploring pathogenesis and phenotype. Pediatr Pulmonol. 2024; 59: 540-551.
- Alonso-Ojembarrena A, Aldecoa-Bilbao V, De Luca D. Imaging of bronchopulmonary dysplasia. Semin Perinatol. 2023; 47: 151812.
- Enzer K G, Baker C D, Wisniewski BL. Bronchopulmonary Dysplasia. Clin Chest Med. 2024; 45: 639-650.
- Jobe AH. Mechanisms of Lung Injury and Bronchopulmonary Dysplasia. Am J Perinatol. 2016; 33: 1076-8.
- Bardanzellu F, Piras C, Atzei A, Neroni P, Fanos V. Early Urinary Metabolomics in Patent Ductus Arteriosus Anticipates the Fate: Preliminary Data. Front Pediatr. 2020; 8: 613749.
- Thébaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers. 2019; 5: 78.
- Moschino L, Bonadies L, Baraldi E. Lung growth and pulmonary function after prematurity and bronchopulmonary dysplasia. Pediatr Pulmonol. 2021; 56: 3499-3508.
- Wu KY, Jensen EA, White AM, Wang Y, Biko DM, et al. Characterization of Disease Phenotype in Very Preterm Infants with Severe Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2020; 201: 1398-1406.
- Soini Y. Claudins in lung diseases. Respir Res. 2011; 12: 70.
- Bonadies L, Zaramella P, Porzionato A, Perilongo G, Muraca M, Baraldi E. Present and Future of Bronchopulmonary Dysplasia. J Clin Med. 2020; 9:1539.
- Abman SH, Bancalari E, Jobe A. The Evolution of Bronchopulmonary Dysplasia after 50 Years. Am J Respir Crit Care Med. 2017; 195: 421-424.
- Xing W, He W, Li X, Chen J, Cao Y, Zhou W, et al. Early severity prediction of BPD for premature infants from chest X-ray images using deep learning: A study at the 28th day of oxygen inhalation. Comput Methods Programs Biomed. 2022; 221: 106869.
- Bonadies L, Cavicchiolo ME, Priante E, Moschino L, Baraldi E. Prematurity and BPD: what general pediatricians should know. Eur J Pediatr. 2023; 182: 1505-1516.
- Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J Pediatr. 2018; 197: 300-308.
- Krishnan M, Dassios T, Bothamley Z, Haque S, Watson C, et al. Prediction of bronchopulmonary dysplasia by the chest radiographic thoracic area on day one in infants with exomphalos. J Perinat Med. 2024; 52: 429-432.
- Ruan Q, Wang J, Shi Y. Clinical Characteristics and Outcomes Until 2 Years of Age in Preterm Infants with Typical Chest Imaging Findings of Bronchopulmonary Dysplasia: A Propensity Score Analysis. Front Pediatr. 2021; 9: 712516.
- Cassady S J, Lasso-Pirot A, Deepak J. Phenotypes of Bronchopulmonary Dysplasia in Adults. Chest. 2020; 158: 2074-2081.
- Kurepa D, Zaghloul N, Watkins L, Liu J. Neonatal lung ultrasound exam guidelines. J Perinatol. 2018; 38: 11-22.
- Raimondi F, Yousef N, Migliaro F, Capasso L, De Luca D. Point-of-care lung ultrasound in neonatology: classification into descriptive and functional applications. Pediatr Res. 2021; 90: 524-531.
- Goffi A, Kruisselbrink R, Volpicelli G. The sound of air: point-of-care lung ultrasound in perioperative medicine. Can J Anaesth. 2018; 65: 399-416.
- Bobillo-Perez S, Girona-Alarcon M, Rodriguez-Fanjul J, Jordan I, Balaguer Gargallo M. Lung ultrasound in children: What does it give us? Paediatr Respir Rev. 2020; 36: 136-141.
- Aldecoa-Bilbao V, Velilla M, Teresa-Palacio M, Esponera CB, et al. Lung Ultrasound in Bronchopulmonary Dysplasia: Patterns and Predictors in Very Preterm Infants. Neonatology. 2021; 118: 537-545.
- Escourrou G, De Luca D. Lung ultrasound decreased radiation exposure in preterm infants in a neonatal intensive care unit. Acta Paediatr. 2016; 105: e237-9.
- Schapka E, Gee J, Cyrus JW, Goldstein G, Greenfield K, et al. Lung Ultrasound versus Chest X-Ray for the Detection of Fluid Overload in Critically Ill Children: A Systematic Review. J Pediatr Intensive Care. 2021; 11: 177-182.
- Garcia MVF, Wiesen J, Dugar S, Adams JR, Bott-Silverman C, Moghekar A, et al. Lung ultrasonography derived B-line scores as predictors of left ventricular end-diastolic pressure and pulmonary artery wedge pressure. Respir Med. 2023; 219: 107415.
- Fontijn S, Balink SJA, Bonte M, Andrinopoulou ER, Duijts L, et al. Chest computed tomography in severe bronchopulmonary dysplasia: Comparing quantitative scoring methods. Eur J Radiol. 2023; 169: 111168.
- Rosenow T, Oudraad MC, Murray CP, Turkovic L, Kuo W, et al. PRAGMA-CF. A Quantitative Structural Lung Disease Computed Tomography Outcome in Young Children with Cystic Fibrosis. Am J Respir Crit Care Med. 2015; 191: 1158-65.
- Spielberg DR, Walkup LL, Stein JM, Crotty EJ, Rattan MS, et al. Quantitative CT scans of lung parenchymal pathology in premature infants ages 0-6 years. Pediatr Pulmonol. 2018; 53: 316- 323.
- Vanhaverbeke K, Van Eyck A, Van Hoorenbeeck K, De Winter B, Snoeckx A, Mulder T, et al. Lung imaging in bronchopulmonary dysplasia: a systematic review. Respir Med. 2020; 171: 106101.
- Higano NS, Bates AJ, Gunatilaka CC, Hysinger EB, Critser PJ, et al. Bronchopulmonary dysplasia from chest radiographs to magnetic resonance imaging and computed tomography: adding value. Pediatr Radiol. 2022; 52: 643-660.
- Savoia P, Jayanthi SK, Chammas MC. Focused Assessment with Sonography for Trauma (FAST). J Med Ultrasound. 2023; 31: 101-106.
- Walkup LL, Tkach JA, Higano NS, Thomen RP, Fain SB, et al. Quantitative Magnetic Resonance Imaging of Bronchopulmonary Dysplasia in the Neonatal Intensive Care Unit Environment. Am J Respir Crit Care Med. 2015; 192: 1215-22.
- Critser PJ, Higano NS, Tkach JA, Olson ES, Spielberg DR, et al. Cardiac Magnetic Resonance Imaging Evaluation of Neonatal Bronchopulmonary Dysplasia-associated Pulmonary Hypertension. Am J Respir Crit Care Med. 2020; 201: 73-82.
- Förster K, Ertl-Wagner B, Ehrhardt H, Busen H, Sass S, Pomschar A, et al. Altered relaxation times in MRI indicate bronchopulmonary dysplasia. Thorax. 2020; 75: 184-187.
- Sheikh K, Coxson H O, Parraga G. This is what COPD looks like. Respirology. 2016; 21: 224-236.
- Chiumello D, Coppola S, Catozzi G, Danzo F, Santus P, Radovanovic D. Lung Imaging and Artificial Intelligence in ARDS. J Clin Med. 2024; 13: 305.
- Ramirez Z R, Ghi T. Use of artificial intelligence and deep learning in fetal ultrasound imaging. Ultrasound Obstet Gynecol. 2023; 62: 185-194.
- Fiorentino MC, Villani FP, Di Cosmo M, Frontoni E, Moccia S. A review on deep-learning algorithms for fetal ultrasound-image analysis. Med Image Anal. 2023; 83: 102629.
- Chou HY, Lin YC, Hsieh SY, Chou HH, Lai CS, Wang B, et al. Deep Learning Model for Prediction of Bronchopulmonary Dysplasia in Preterm Infants Using Chest Radiographs. J Imaging Inform Med. 2024; 37: 2063-2073.
- Williams J, Ahlqvist H, Cunningham A, Kirby A, Katz I, et al. Validated respiratory drug deposition predictions from 2D and 3D medical images with statistical shape models and convolutional neural networks. PLoS One. 2024; 19: e0297437.
- Zhang M, Wen G, Zhong J, Chen D, Wang C, et al. MLP-Like Model with Convolution Complex Transformation for Auxiliary Diagnosis Through Medical Images. IEEE J Biomed Health Inform. 2023; 27: 4385-4396.
- Zhao R X, Shi J, Li X. QKSAN: A Quantum Kernel Self-Attention Network. IEEE Trans Pattern Anal Mach Intell. 2024; 46: 10184- 10195.
- Hwang JK, Kim DH, Na JY, Son J, Oh YJ, et al. Two-stage learning- based prediction of bronchopulmonary dysplasia in very low birth weight infants: a nationwide cohort study. Front Pediatr. 2023; 11: 1155921.
- Wang C, Ma X, Xu Y, Chen Z, Shi L, Du L. A prediction model of pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Front Pediatr. 2022; 10: 925312.
- Akhter Y, Singh R, Vatsa M. AI-based radiodiagnosis using chest X-rays: A review. Front Big Data. 2023; 6: 1120989.
- Shah STH, Shah SAH, Khan II, Imran A, Shah SBH, et al. Data-driven classification and explainable-AI in the field of lung imaging. Front Big Data. 2024; 7: 1393758.
- Meng L, Zhao D, Yang Z, Wang B. Automatic display of fetal brain planes and automatic measurements of fetal brain parameters by transabdominal three-dimensional ultrasound. J Clin Ultrasound. 2020; 48: 82-88.
- Østvik A, Smistad E, Aase SA, Haugen BO, Lovstakken L. Real-Time Standard View Classification in Transthoracic Echocardiography Using Convolutional Neural Networks. Ultrasound Med Biol. 2019; 45: 374-384.
- Baumgartner CF, Kamnitsas K, Matthew J, Fletcher TP, Smith S, et al. SonoNet: Real-Time Detection and Localisation of Fetal Standard Scan Planes in Freehand Ultrasound. IEEE Trans Med Imaging. 2017; 36: 2204-2215.
- Narang A, Bae R, Hong H, Thomas Y, Surette S, et al. Utility of a Deep-Learning Algorithm to Guide Novices to Acquire Echocardiograms for Limited Diagnostic Use. JAMA Cardiol. 2021; 6: 624-632.
- Fischer AM, Varga-Szemes A, van Assen M, Griffith LP, Sahbaee P, et al. Comparison of Artificial Intelligence-Based Fully Automatic Chest CT Emphysema Quantification to Pulmonary Function Testing. AJR Am J Roentgenol. 2020; 214: 1065-1071.
- Paik SH, Jin GY. Using Artificial Intelligence Software for Diagnosing Emphysema and Interstitial Lung Disease. J Korean Soc Radiol. 2024; 85: 714-726.
- Loi B, Vigo G, Baraldi E, Raimondi F, Carnielli VP, et al. Lung Ultrasound to Monitor Extremely Preterm Infants and Predict Bronchopulmonary Dysplasia. A Multicenter Longitudinal Cohort Study. Am J Respir Crit Care Med. 2021; 203: 1398-1409.
- Higano NS, Spielberg DR, Fleck RJ, Schapiro AH, Walkup LL, et al. Neonatal Pulmonary Magnetic Resonance Imaging of Bronchopulmonary Dysplasia Predicts Short-Term Clinical Outcomes. Am J Respir Crit Care Med. 2018; 198: 1302-1311.
- Mairhörmann B, Castelblanco A, Häfner F, Koliogiannis V, Haist L, et al. Automated MRI Lung Segmentation and 3D Morphologic Features for Quantification of Neonatal Lung Disease. Radiol Artif Intell. 2023; 5: e220239.
- Parraga G, Sharma M. Don’t Forget the Kids!: Novel Pulmonary MRI and AI of Neonatal Lung Disease. Radiol Artif Intell, 2023; 5: e230400.
- Wang G, Ye J C, De Man B. Deep learning for tomographic image reconstruction. Nature Machine Intelligence. 2020; 2: 737-748.
- Li JW, Sheng DL, Chen JG, You C, Liu S, et al. Artificial intelligence in breast imaging: potentials and challenges. Phys Med Biol. 2023; 68.
- Tozzo P, Angiola F, Gabbin A, Politi C, Caenazzo L. The difficult role of Artificial Intelligence in Medical Liability: to err is not only human. Clin Ter. 2021; 172: 527-528.
- Vearrier L, Derse AR, Basford JB, Larkin GL, Moskop JC. Artificial Intelligence in Emergency Medicine: Benefits, Risks, and Recommendations. J Emerg Med. 2022; 62: 492-499.
- Maliha G, Gerke S, Cohen IG, Parikh RB. Artificial Intelligence and Liability in Medicine: Balancing Safety and Innovation. Milbank Q. 2021; 99: 629-647.
- Balsano C, Burra P, Duvoux C, Alisi A, Piscaglia F, et al. Artificial Intelligence and liver: Opportunities and barriers. Dig Liver Dis. 2023; 55: 1455-1461.
- Dai D, Chen H, Dong X, Chen J, Mei M, et al. Bronchopulmonary Dysplasia Predicted by Developing a Machine Learning Model of Genetic and Clinical Information. Front Genet. 2021; 12: 689071.
- Baker CD, Abman SH. Impaired pulmonary vascular development in bronchopulmonary dysplasia. Neonatology. 2015; 107: 344-351.