Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
The feasibility of physical therapy in a patient with Parkinson’s Disease and Myasthenia Gravis: A case report
Haoyu Xie2; Jung Hung Chien2; Zhiqin Xu1*
1 Department of Rehabilitation Medicine, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China.
2 Division of Physical Therapy Education, College of Allied Health Professions, University of Nebraska Medical Center, 984420 Nebraska Medical Center, Omaha, Nebraska, USA.
*Corresponding Author: Zhiqin Xu
Department of Rehabilitation Medicine, The First
Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China.
Email: xuzhqin@mail.sysu.edu.cn
Received : Oct 27, 2021
Accepted : Dec 02, 2021
Published : Dec 09, 2021
Archived : www.jcimcr.org
Copyright : © Xu Z (2021).
Abstract
The occurrence for patients who have the co-existence of Parkinson’s Disease (PD) and Myasthenia Gravis (MG) is rare. This study attempted to investigate the feasibility of physical therapy on a patient with PD and MG. A 56-year-old male patient had MG for 42 years and PD for 10 years. He received physical therapy, including the 5-minute gait training and balance training, twice per week for 8 weeks. Quantitative Myasthenia Gravis Score (QMGs), Modified Barthel Index (MBI), Berg Balance Scale (BBS), and Parkinson’s Disease Questionnaire (PDQ-39) were used at baseline and post-intervention evaluation. The Unified Parkinson’s Disease Rating Scale (UPDRS) activities of daily living part (UPDRS-II) and motor part (UPDRS-III) were performed every two weeks until the end of intervention. After the intervention of 8 weeks, QMGs decreased by 3 (-43%), BBS increased by 4 (9%), MBI increased by 12 (14%), and PDQ-39 decreased by 5 (-11%). UPDRS-II, UPDRS-III and total UPDRS gradually decreased by 2 (-14%), 6 (-18%), and 8 (-17%) respectively during the intervention. With improved results of clinical scales, motor and ADL functions of this patient were enhanced. Our results suggested that physical therapy on the patient with PD and MG was feasible and effective, which deserves more attention and warrants further research.
Keywords: Parkinson’s disease; Myasthenia gravis; physical therapy; UPDRS.
Citation: Xie H, Chien J, Xu Z. The feasibility of physical therapy in a patient with Parkinson’s Disease and Myasthenia Gravis: A case report. J Clin Images Med Case Rep. 2021; 2(6): 1463.
Introduction
Parkinson’s Disease (PD) is a progressive neurodegenerative disease, which is characterized by the disruption of the neurotransmitter system with the loss of dopaminergic neurons [1]. Myasthenia Gravis (MG) as prototypic autoimmune disease shows as muscle weakness due to the neuromuscular junction disorder. It is usually caused by specific antibodies binding to the acetylcholine receptor, which leads to the decreased amount of acetylcholine activated the receptor and repetitive weakness of muscles [2]. The co-existence of PD and MG in an individual is exceptionally rare. To our knowledge, only 29 cases diagnosed as PD and MG have been reported since 1987. The common symptoms of patients with PD and MD mainly include tremor, rigidity, bradykinesia, ptosis, diplopia, and head drop [3]. Previous study mainly focused on the drug therapy for this kind of patient, but it has little benefits on alleviating motor symptoms and improving motor function of the patient [3]. Physical therapy, as a non-drug intervention, has been proven as effective in patients with PD or MG, and plays an important role to compensate the limitation of drug therapy [4].
Recently, gait training and balance training as non-drug interventions for PD has gained more and more recognition. Gait training is aimed to promote patient’s standing and walking ability. According to the evidence-based analysis, gait training was a common intervention and proven as effective in improving stride length and balance while walking for PD patients [4]. Another meta-analysis showed that gait training with external auditory cues could enhance gait velocity and step length among PD patients further, when compared with gait training without cues or with visual cues only [5]. Furthermore, balance training, such as Single Leg Stance (SLS) and weight shifting, is also important for PD patients to improve postural control and prevent the falls [6]. A previous randomized controlled trial showed that SLS training was more effective in improving dynamic balance with higher Berg Balance Scale (BBS) scores than resistance training in PD patients [7]. Additionally, a few pilot studies also verified the feasibility of the SLS training on improving balance function in patients with mild MG [8,9]. However, the evidence about the feasibility of physical therapy on patients with PD and MG is still unknown.
As far as we know, in previous studies, no one had applied physical therapy on individuals diagnosed with PD and MG. The investigation of physical therapy as the non-drug intervention in this kind of patients is needed. In the present study, we reported a new case diagnosed as PD and MG, and applied 8-week physical therapy on this patient to investigate the feasibility and effectiveness of physical therapy. We hypothesized that physical therapy would improve physical capacity and Activity of Daily Living (ADL) function.
Case description
A 56-year-old male patient, who developed bilateral ptosis and double vision since 1977, was diagnosed as MG with the neostigmine test (+) in the same year. The muscle weakness occurred mostly at the end of the day and decreased after rests. The patient was treated long-term with Pyridostigmine, and the ptosis alleviated. In 2009, the patient gradually developed bradykinesia and the decreased swing on his left upper limb when walking. The resting tremor was not observed. In a month, he was diagnosed as PD by a neurologist. After the diagnosis, the patient was being treated with Rasagiline 1 mg q.d. and Pyridostigmine 60 mg t.i.d., then the symptoms of PD were obviously alleviated. In March 2011, MG’s conditions aggravated with worse bilateral ptosis and double vision, dysarthria, and dysphagia. Then the immunoregulatory therapy was used and the symptoms of MG relieved. After diagnosed as thymus hyperplasia via Magnetic Resonance Imaging (MRI), an extensive thymectomy was performed under endotracheal anesthesia on December 20th, 2011. After the surgery, the patient was treated with Pyridostigmine 60 mg t.i.d. and Prednisolone 15 mg q.d. Bradykinesia and the resting tremor remained. In the case of stable control by drug therapy, the physician thought that it was necessary for the patient to improve motor function via nondrug therapy. Therefore, the patient was transferred to the Department of Rehabilitation Medicine.
This case study was approved by the ethical committee of the First Affiliated Hospital, Sun Yat-sen University (Ethics no.: IIT-2020-145). This patient was informed of the study protocol, and all questions were explained in detail. Informed written consent was obtained prior to data collection. This patient was free to withdraw from the study at any time without providing a reason. The Declaration of Helsinki was strictly followed throughout the course of the study.
Physical therapy protocol
From July 2019 to September 2019, the patient received physical therapy in the morning twice per week for 8 weeks, totaling 16 sessions. Each session of intervention lasted 45 to 60 minutes. A licensed physical therapist who was not involved in the study instructed the patient to complete the intervention. The physical therapy included the following two items:
(1) The 5-minute gait training: There were 14 auxiliary lines on the ground as visual cues to provide feedback for the patient. Each line was one meter apart, and thirteen meters in length in total. The patient was asked to walk along the auxiliary lines with a minimum number of steps, then he turned back and repeated for 5 minutes. A physiotherapist monitored the patient to keep him safe and provided auditory cues, such as “Increase swing arms” and “Focus on your steps”. The gait training was performed 5 times. There was a 3-minute break between every two sessions of gait training. If the patient felt too fatigue to continue on, the gait training would stop, and he was allowed to have a sufficient rest.
(2) Balance training: The balance training included the 30-second SLS and the Center of Mass (COM) shifting training. In the SLS training, the patient was instructed to lift one foot up to the knee and keep balance as long as possible. After 30 seconds, he would return to standing on both feet and have a break of 10 seconds. Then the patient alternated to lift the other foot and repeated. The SLS training was performed with 5 repetitions on each side. In the COM shifting training, the patient stood on both feet and was instructed to lean forward or sidewise to reach the physical therapist’s hands. The COM shifting training was repeated for 5 repetitions. The physical therapist provided necessary assistance to prevent falls.
If motor fluctuation occurred during the intervention period, the patient would be asked to stop the current training and have a sufficient rest. A physical therapist would perform passive Range Of Motion (ROM) training and Proprioceptive Neuromuscular Stimulation (PNF) training to maintain ROM of joints and decrease myotonus.
Evaluation
All evaluations were conducted in a separate physical therapy room of the First Affiliated Hospital, Sun Yat-sen University. The primary outcome, the Unified Parkinson’s Disease Rating Scale (UPDRS) activities of daily living part (UPDRS-II) and motor part (UPDRS-III), was measured every two weeks. All UPDRS assessments were conducted on Friday per two weeks, and patient was required to not do vigorous physical activities before the evaluation. Secondary outcome measures included the Quantitative Myasthenia Gravis Score (QMGs), the Modified Barthel Index (MBI), BBS, and Parkinson’s Disease Questionnaire (PDQ-39).
The UPDRS is the common evaluation scale with 4 parts (I: mentation, behavior, mood; II: activities of daily living, III: motor, IV: motor complications) and 42 items in total [10]. Parts I to III were scored on a 0-4 rating scale, and part IV was scored with yes and no ratings. These 4 parts provided a systematic and comprehensive evaluation of physical capacity, symptoms, and daily participation for patients with PD. In this study, UPDRS-II and UPDRS-III were used to focus on assessing the motor and ADL functions of this patient. The full scores for UPDRS-II and III were 52 and 108 points, 160 points in total. Higher scores indicate increased severity.
The QMGs is a 13-item scale used to quantify the severity of MG [11]. The QMGs measures ocular, bulbar, respiratory, and limb function, ranging from 0 (no myasthenic findings) to 39 (maximal myasthenic deficits). The MBI is a common clinical assessment tool for ADL function with 10 items, 100 points in total [12]. Higher scores indicate better function. Previous study has proven the feasibility of MBI on evaluating the ADL function of patients with PD [12]. The BBS consisting of 14 items requires individuals to maintain or assume positions of varying difficulty [13]. The ability to perform each task is graded from 0 to 4, with a total possible score of 56. Higher scores indicate better balance. The PDQ-39 is the self-rating scale with 39 items for PD, which involves mobility, emotion, social support, and cognition sections [14]. Lower scores indicate better quality of life.
These variables were selected to assess the symptoms of MG and PD, ADL function, balance, and the quality of life in this patient. More than one outcome variables were selected as we aimed to comprehensively investigate the effects of physical therapy on the physical capacity and ADL participation of this individual with PD and MG.
Results
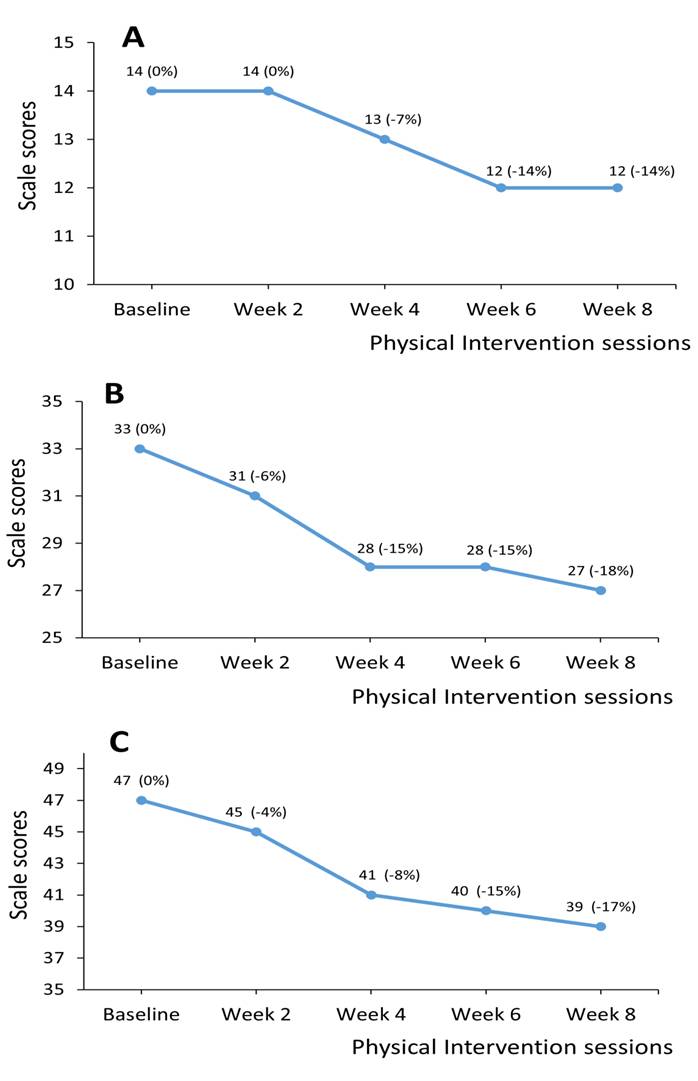
The patient completed all 16 sessions of physical therapy. No motor fluctuation or adverse events occurred during the intervention. Table 1 showed the evaluation results of scales used. At baseline evaluation, QMGs was 7, MBI score was 83, and BBS score was 45. UPDRS-II and III scores were 14 and 33, respectively, and total score was 47. After the intervention, QMGs declined by 3 (-43%), MBI score increased 12 (14%), and BBS score increased by 4 (9%). UPDRS-II and III decreased by 2 (-14%) and 6 (-18%), respectively, and UPDRS total score decreased by 8 (-17%). PDQ-39 showed a decrease of 5 (-11%) after the intervention. Figure 1 showed the change tendency of UPDRS-II, III, and total scores during the intervention.
Table 1: Results of clinical scales.
|
Baseline |
Post-intervention |
Changea (%b) |
|
QMGs |
7 |
4 |
-3 (-43%) |
|
BBS |
45 |
49 |
4 (9%) |
|
MBI |
83 |
95 |
12 (14%) |
|
PDQ-39 |
47 |
42 |
-5 (-11%) |
|
UPDRS |
|
|
|
|
Total |
47 |
39 |
-8 (-17%) |
|
II |
14 |
12 |
-2 (-14%) |
|
III |
33 |
27 |
-6 (-18%) |
|
a Change score from baseline to post-intervention. bThe percentage of change compared with baseline.
Discussion
In this case study, a patient with the co-existence of PD and MG received physical therapy for 8 weeks. The results supported our hypothesis that physical therapy is feasible and effective to improve physical capacity and ADL function of individuals with PD and MG. To our knowledge, it was the first time that physical therapy was applied on the patient with PD and MG. Furthermore, the patient spontaneously reported that he was willing to participate in the physical therapy and looked forward to continuing the training at home after the study. We believe that his motivation and positive attitude were significant components of his successful participation.
Physical therapy improved physical capacity and ADL function in the patient with PD and MG
Based on our results, this patient exhibited improvements in motor and ADL functions in terms of the decreased UPDRS-II and III scores after the intervention. This finding is accord with previous studies that UPDRS motor and ADL scores significantly declined among PD patients who received non-aerobic gait exercises, when compared with PD patients taking only normal physical activity [15,16]. According to a previous clinical study, the Minimal Clinically Important Difference (MCID) for UPDRSII was-1.8 and -2.3, and for UPDRS-III -5.2 and -6.5 [17]. The changes of UPDRS in this study were consistent with it, and suggested that the motor and ADL function had a clinically meaningful improvement after the physical therapy. In addition, a meta-analysis reviewed 39 studies about PD and showed that there was a mean decrease of 1.36 (95% confidence interval 0.30 to 2.41) in UPDRS-II scores and 5.01 (95%CI 3.72 to 6.30) in UPDRS-III respectively among PD patients after receiving physical therapy including gait and balance exercise [18]. Our results matched the ranges of UPDRS scores of this meta-analysis. It indicated that physical therapy in this study had a significant facilitation on this patient, although he also had MG, and the comorbid MG didn’t disturb the rehabilitation for PD in this study. But compared to patients with only PD, the existence of muscle weakness and decreased exercise tolerance in patient with PD and MG needs extra attention to avoid the adverse event [19].
In addition, decreased QMGs indicated that physical therapy also has an improvement effect on the symptoms of MG. However, previous studies about physical exercise on MG patients were limited, and most of them focused on the recovery of muscle strength [20]. We speculated that the alleviation of the symptoms of MG may be due to the 5-minute gait training that functioned as a mild endurance training to enhance exercise capacity of this patient, which is consistent with a previous case study [21]. The increased scores of MBI added to the evidence that physical therapy could improve the ADL function, and the percentage of change in MBI (14%) was similar to that in UPDRS-II (14%). In addition, there was an increase of 4 points in BBS after the intervention, which matched the mean difference of 3.71 (95%CI 2.30 to 5.11) from a meta-analysis [18]. However, based on a published methodological research, the minimal detective change (MDC) and MCID of BBS were 6.3 and 7 points, respectively [22]. The increase of BBS in this study didn’t reach the minimal important change. It may be due to the short intervention duration. A previous randomized controlled trial with 21-session balance training on patients with PD showed an increase of 7.0 points on BBS [23]. So, a further increase on BBS in this case is expected after a longer period of physical therapy. Decreased score of 5 points in PDQ-39 indicated that from the patient’s perspective, there was a significant improvement in the quality of life when the clinically important change in PDQ39 was determined as 1.6 points [24]. Although from the patient’s view, the physical therapy had an important change in his life, we couldn’t rule out that it may be due to the Hawthorne effect that overstated the effect of physical therapy [25].
The possible ceiling effect of physical therapy for patients with PD
From Figure 1, we observed a plateau in UPDRS-III from week 8 to 10. It indicated that the effect of physical therapy on motor function of the patient decreased after the first four weeks of physical therapy. This finding is consistent with a previously published study, whose results showed that the UPDRS motor scores of PD patients who received the LSVT® BIG therapy had a significant decrease in the first four weeks, and then the decline obviously moderated [26]. We speculated that the effectiveness of physical therapy on improving motor function of PD patients may have a ceiling effect, and the recovery of motor function would slow down after receiving the same intervention for four weeks. Therefore, physical therapists may properly increase the difficulty of training or select another exercise program to maintain the continuous improvement of motor function in PD patients every four weeks in the clinical practice. Furthermore, we also observed that the UPDRS-II score remained at 14 points as pre-intervention evaluation after the first two weeks of intervention phase, then it showed a decrement in the next four weeks. We speculated that the improvement of ADL function in the patient had ahysteresis of about 2 weeks, compared with the motor function. As the ADL function is more complex and comprehensive than the general motor function, the recovery of ADL function may be based on the improvement of motor function. A previous study from Nackaerts et al. [27] verified the enhanced neural connectivity of cortical network from supplementary motor area to primary motor cortex among patients with PD after 6-week physical therapy. It suggested that motor task training played an important role in the transfer of motor learning to skill acquisition [27]. Therefore, we speculated that it may take about 2 weeks for the promotion of physical capacity to transfer and integrate to the improvement of ADL function in the cortical network. However, further research is needed to prove our deduction.
Limitations
Limitations of this case study include the following two aspects. First, this patient received physical therapy twice per week in the hospital, but we didn’t record other physical activities that he participated in at other times. These unrecorded physical activities may also contribute to the improvement of this patient. Second, the intervention period in this study was only 8 weeks. In the future, a longer period of intervention is needed to observe the long-term effect of physical therapy on patients with PD and MG.
Conclusion
In this case study, an 8-week physical therapy consisting of the 5-minute gait training and balance training was performed in a patient with PD and MG. To our knowledge, this is the first report to use physical therapy on this kind of rare patient. Our results suggested that physical therapy had a positive effect on motor and ADL functions of this patient.
Declarations
Conflicts of interest: The authors declares that there is no conflict of interest regarding the publication of this paper.
Acknowledgements: The authors would like to thank Xi Chen, PhD, for reviewing and providing comments for this manuscript. And authors appreciate the patient for his participation.
Author contributions: ZX, and HX recruited the participant and performed theexperiments. HX and JC wrote the paper. ZX made contributions to the experiments. HX, JC, and ZX reviewed and edited the manuscript. All authorshad read and approved the manuscript.
Funding: There was no funding for this research.
References
- Fox SH, Katzenschlager R, Lim SY, et al. International Parkinson and movement disorder society evidence based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018; 33: 1248-1266.
- Hehir MK, Silvestri NJ. Generalized Myasthenia Gravis: Classification, Clinical Presentation, Natural History, and Epidemiology. Neurol Clin. 2018; 36: 253-260.
- Odajiu I, Davidescu EI, Mitu C, Popescu BO. Patients with Parkinson’s Disease and Myasthenia Gravis-A Report of Three New Cases and Review of the Literature. Medicina (Kaunas). 2019; 56: 5.
- Keus SH, Bloem BR, Hendriks EJ, Bredero-Cohen AB, et al. Practice Recommendations Development Group. Evidence based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord. 2007; 22: 451-600.
- Spaulding SJ, Barber B, Colby M, Cormack B, Mick T, et al. Cueing and gait improvement among people with Parkinson’s disease: A meta-analysis. Arch Phys Med Rehabil. 2013; 94: 562-570.
- Mak MKY, Wong-Yu ISK. Exercise for Parkinson’s disease. Int Rev Neurobiol. 2019; 147: 1-44.
- Santos SM, da Silva RA, Terra MB, Almeida IA, de Melo LB, et al. Balance versus resistance training on postural control in patients with Parkinson’s disease: A randomized controlled trial. Eur J Phys Rehabil Med. 2017; 53: 173-183.
- Rahbek MA, Mikkelsen EE, Overgaard K, Vinge L, Andersen H, et al. Exercise in myasthenia gravis: A feasibility study of aerobic and resistance training. Muscle Nerve. 2017; 56: 700-709.
- Westerberg E, Molin CJ, Lindblad I, Emtner M, Punga AR, et al. Physical exercise in myasthenia gravis is safe and improves neuromuscular parameters and physical performance-based measures: A pilot study. Muscle Nerve. 2017; 56: 207-214.
- Barohn RJ, McIntire D, Herbelin L, Wolfe GI, Nations S, et al. Reliability testing of the quantitative myasthenia gravis score. Ann N Y Acad Sci. 1998; 841: 769-72.
- Taghizadeh G, Martinez-Martin P, Meimandi M, et al. Barthel Index and modified Rankin Scale: Psychometric properties during medication phases in idiopathic Parkinson disease. Ann Phys Rehabil Med. 2020; 63: 500-504.
- Downs S. The Berg Balance Scale. J Physiother. 2015; 61: 46.
- Martínez-Martín P, Gil-Nagel A, Gracia LM, Gómez JB, MartínezSarriés J, et al. Unified Parkinson’s Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord. 1994; 9: 76-83.
- Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing. 1997; 26: 353-357.
- Sage MD, Almeida QJ. Symptom and gait changes after sensory attention focused exercise vs aerobic training in Parkinson’s disease. Mov Disord. 2009; 24: 1132-1138.
- Comella CL, Stebbins GT, Brown-Toms N, Goetz CG. Physical therapy and Parkinson’s disease: A controlled clinical trial. Neurology. 1994; 44: 376-378.
- Hauser RA, Gordon MF, Mizuno Y, et al. Minimal clinically important difference in Parkinson’s disease as assessed in pivotal trials of pramipexole extended release. Parkinsons Dis. 2014; 2014 :467131.
- Tomlinson CL, Patel S, Meek C, et al. Physiotherapy intervention in Parkinson’s disease: Systematic review and meta-analysis. BMJ. 2012; 345: e5004.
- Elsais A, Johansen B, Kerty E. Airway limitation and exercise intolerance in well regulated myasthenia gravis patients. Acta Neurol Scand Suppl. 2010; 12-17.
- Anziska Y, Inan S. Exercise in neuromuscular disease. Semin Neurol. 2014; 34: 542-56.
- Lucia A, Maté-Muñoz JL, Pérez M, Foster C, Gutiérrez-Rivas E, Arenas J. Double trouble (McArdle’s disease and myasthenia gravis): how can exercise help?. Muscle Nerve. 2007; 35: 125- 128.
- Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A, et al. Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys Ther. 2013; 93: 158-167.
- Smania N, Corato E, Tinazzi M, Stanzani C, Fiaschi A, Girardi P, et al. Effect of balance training on postural instability in patients with idiopathic Parkinson’s disease. Neurorehabil Neural Repair. 2010; 24: 826-834
- Margolius A, Cubillos F, He Y, et al. Predictors of clinically meaningful change in PDQ-39 in Parkinson’s disease. Parkinsonism Relat Disord. 2018; 56 : 93-97.
- Morberg BM, Malling AS, Jensen BR, Gredal O, Wermuth L, et al. The Hawthorne effect as a pre-placebo expectation in Parkinsons disease patients participating in a randomized placebocontrolled clinical study. Nord J Psychiatry. 2018; 72: 442-446.
- Ebersbach G, Ebersbach A, Edler D, et al. Comparing exercise in Parkinson’s disease the Berlin LSVT®BIG study. Mov Disord. 2010; 25: 1902-1908.
- Nackaerts E, Michely J, Heremans E, et al. Training for Micrographia Alters Neural Connectivity in Parkinson’s Disease. Front Neurosci. 2018; 12: 3.