Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
Pemetrexed-cisplatin combined with antiangiogenesis drug in recurrent and metastatic cervical carcinoma: A case report and literature review
Hui Yang*; Zijie Mei; Shaoxing Sun; Hui Qiu*
Department of Radiation and Medical Oncology, Hubei Key Laboratory of Tumor Biological Behaviors, Hubei Cancer Clinical Study Center, Zhongnan Hospital of Wuhan University, Wuhan, China.
*Corresponding Author: Hui Yang & Hui Qiu
Department of Radiation and Medical Oncology,
Hubei Key Laboratory of Tumor Biological Behaviors,
Hubei Cancer Clinical Study Center, Zhongnan Hospital of Wuhan University, 160 Donghu Road, Wuhan
430071, China.
Email: huiyangwhu@whu.edu.cn &
qiuhuiznyy@whu.edu.cn
Received : Nov 13, 2021
Accepted : Jan 03, 2022
Published : Jan 10, 2022
Archived : www.jcimcr.org
Copyright : © Yang H & Qiu H (2022).
Abstract
Pemetrexed is an antifolate agent which has shown activity on various tumors. But no result has been reported on the clinical efficacy and toxicity of pemetrexed combined with antiangiogenesis drug in patients with cervical cancer. Apatinib is an oral antiangiogenesis drug in cervical cancer, which has been reported efficacy and safety in cervical cancer. Here, we present a 50-year-old woman who was diagnosed with recurrent and metastatic cervical adenocarcinoma two years after postoperative adjuvant radiotherapy. After pathology and magnetic resonance imaging confirmed the recurrence and metastasis, the patient was administered pemetrexed 500 mg/m2 and cisplatin 50 mg/m2 on day 1, cycled every three weeks for four cycles. Then add apatinib 500 mg/d from day 1 to day 21 combined with pemetrexed and cisplatin for two cycles, followed by pemetrexed 500 mg/m2 on day 1 combined with apatinib every 21 days per cycle for two cycles. Finally, a single dose of apatinib is a maintenance therapy. According to the Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 standard, she was confirmed as Partial Response (PR). Now, the Progression-Free Survival (PFS) of the patient has been 21 months. No grade III-IV adverse effects have been found. In conclusion, pemetrexed-cisplatin combined with antiangiogenesis drug might be an effective and mild toxic treatment for patients with recurrent or metastatic cervical carcinoma. Further prospective clinical trials are needed to verify this conclusion.
Keywords: cervical cancer; apatinib; pemetrexed-cisplatin; antiangiogenesis.
Citation: Yang H, Mei Z, Sun S, Qiu H. Pemetrexed-cisplatin combined with antiangiogenesis drug in recurrent and metastatic cervical carcinoma: A case report and literature review. J Clin Images Med Case Rep. 2022; 3(1): 1544.
Introduction
Cervical cancer is the most common cancer in the female reproductive system [1]. The Human Papillomavirus (HPV) screening test and HPV vaccine have been used in developing countries, the incidence of cervical cancer has been sharply decreased [2]. But there are nearly 70% of cervical cancers first diagnosis in the local advanced or metastatic disease in the poverty-stricken areas [3]. After effective surgery or concurrent chemoradiotherapy, the five years overall survival rate for these patients is almost 75-85% [4]. However, when the patients are faced with recurrence or metastasis, the treatment options are so limited that the diseases are usually incurable [5].
Pemetrexed is an antifolate agent which has shown activity on various tumors, including lung, breast, ovarian, and head and neck cancers [6]. A phase II trial (GOG-0076GG) was conducted by the Gynecologic Oncology Group (GOG) to evaluate the efficacy and toxicity of pemetrexed plus cisplatin on the first-line treatment for the patients with recurrent or metastatic cervical cancer [6]. The results showed that the treatment might be less toxic but as active as paclitaxel-cisplatin [6]. As paclitaxel-cisplatin can be combined with antiangiogenesis drugs, the pemetrexed-cisplatin plus antiangiogenesis drugs might be appropriate. Though the activity and safety of pemetrexed-cisplatin plus antiangiogenesis drug have been reported in lung cancer and ovarian cancer [7,8], no studies have reported the effect of this treatment in cervical cancer.
The small molecular antiangiogenesis drugs showed efficacy on various cancers as well as in cervical cancer. Apatinib, a highly selective tyrosine kinase inhibitor that targets the vascular endothelial growth factor receptor-2 (VEGFR-2), could block the signal transduction by inhibiting the combination of VEGF and its receptor, and then suppresses tumor angiogenesis [9]. Qiu et al. have reported that apatinib could suppress tumor growth and increase the sensitivity of paclitaxel in cervical cancer [10]. Zhou et al. have registered a clinical trial on the Chinese Clinical Trials Registry (ChiCTR-OIN-17012164), which designed an open-label randomized controlled trial with apatinib for patients with advanced or recurrent cervical cancer [11]. Some studies have reported that apatinib is an effective and less toxic treatment in patients with recurrent cervical cancer [12-18]. But no result has been reported on the clinical efficacy and toxicity of apatinib combined with pemetrexed-cisplatin in patients with cervical cancer. Thus, we reported a unique case of recurrent and metastatic cervical cancer receiving pemetrexed-cisplatin plus apatinib treatment.
Case report
In May 2015, a 46-year old female was admitted to her local hospital. She complained about irregular vaginal bleeding for 1 year. A vaginal discharge examination showed “HPV-16 infection”, colposcopy cervical biopsy showed “chronic cervicitis with erosion, part of the Glandular epithelium showed high grade Cervical Intraepithelial Neoplasia (CIN III)”. On June 4, 2015, extrafascial hysterectomy was performed on the patient, and the intraoperative frozen section revealed “cervical endometrioid adenocarcinoma”. Thus, it was no doubt to change the operation into radical hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymph node dissection. The postoperative pathology showed that moderately differentiated endometrioid adenocarcinoma, which invades both the uterine cavity and cervix. The depth was less than 1/3. The gross specimen was mainly located in the cervix. The vaginal stump, bilateral attachments, and pelvic lymph nodes in all groups of 12 sections showed no cancer invasion. The results of immunohistochemical staining showed PCK (+), CEA (+), P16 (+), vimentin (-), ER (weak +), PR (-), which suggested that the uterus was originated from the cervical epithelial. Thus, according to the FIGO 2009 (International Federation of Gynecology and Obstetrics) criteria, the patient was diagnosed with cervical adenocarcinoma with stage IB1.
As recommended by the NCCN guidelines, the patients in the stage IB1 with neither high-risk factors (positive lymph nodes, positive vaginal stump, and parametrial infiltration) nor intermediate risk factors (vascular tumor emboli, depth of cervical infiltration is greater than half, and tumor size is not less than 4 cm), could not take any adjuvant therapy, just surveillance. However, the surgeon showed that the patients should take adjuvant radiotherapy after the operation. So the patient was referred to our hospital and asked for radiotherapy. The postoperative pathological sections were consulted in the pathology department, and the results were in accordance with the pathology department of the local hospital. Then, exclusion of contraindication, the patient had been performed the pelvic intensity-modulated radiation therapy (total dose=50Gy/25 fractions) and the vaginal brachytherapy (total dose=12Gy/2 fractions) from June 14 to August 40, 2015.
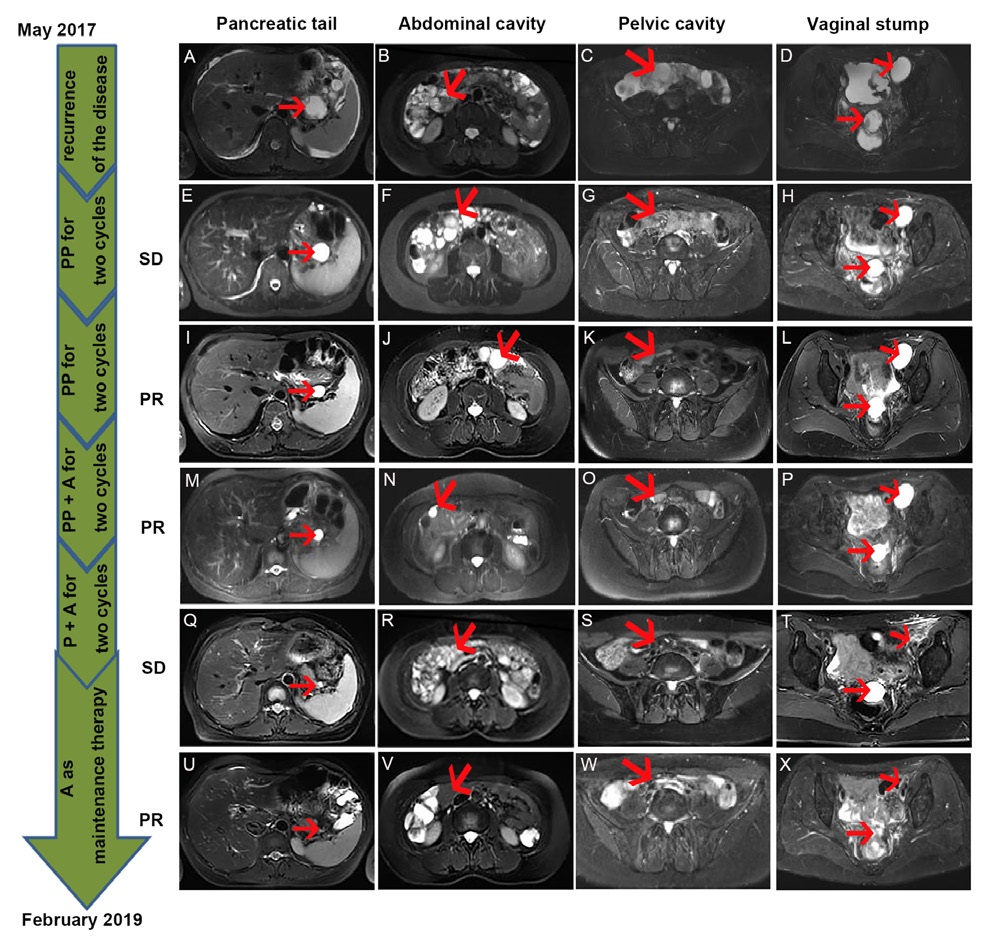
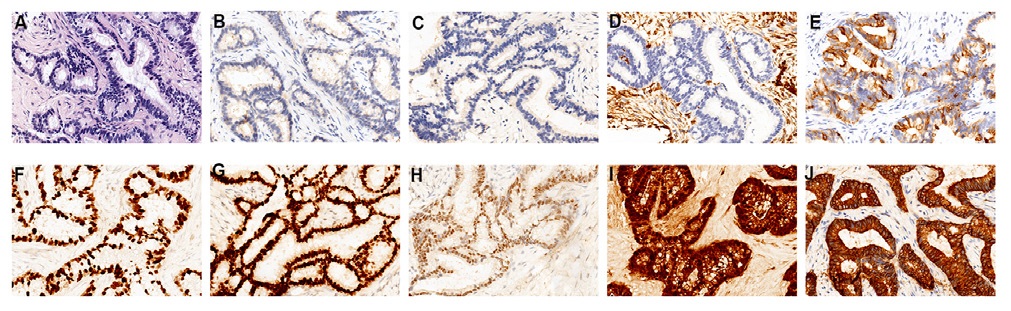
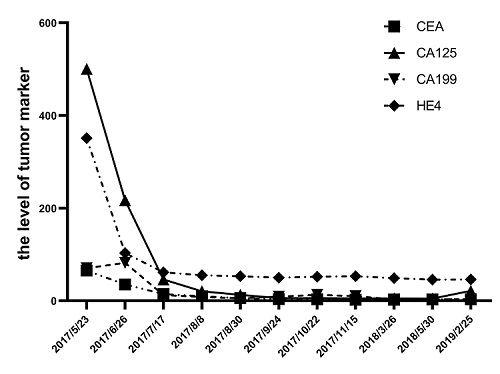
After radiotherapy, the patient was routinely reviewed till May 2017. There was slight pain and discomfort in her abdomen, and she could not relieve it for half a month. So the patient backed to our hospital to perform some routine examinations. The results showed that the Carcinoembryonic Antigen (CEA) 65.13 ng/ml, Glycoprotein Antigen 125 (CA125) 500.3 U/mL, Glycoprotein Antigen 199 (CA199) 70.16 U/mL, Human Epididymis Protein 4 (HE4) 351.2 pmol/L. Abdomen and pelvic Magnetic Resonance Imaging (MRI) suggested that multiple cystic lesions at the pancreatic tail, vaginal stump, abdominal and pelvis cavity, and the pelvic cavity hold a large amount of effusion (Figure 1A-D). The superior doctor considered the recurrence of the disease with extensive pelvic cavity metastasis. The pathological biopsy report of the rectal mass and the lower abdominal mass showed many inflammatory cells and a small number of heterogeneous cell clusters, combined with the medical history, considered metastatic adenocarcinoma. Immunohistochemistry showed that tumor cells were CDX2(-), CK(+), ER(+), P16(+), PAX8(+), PR(-), VIMENTIN(-), CK20 scattered positive, Ki-67 ( 80%) (Figure 2A-J). Reviewed the patient’s previous postoperative pathology report, it was consistent with current histological features. According to these, the patient might be with extensive metastases and recurrence of cervical cancer.
Chemotherapy combined with angiogenesis blockade is the first-line treatment for recurrent and metastatic cervical cancer. The doctor recommended the patient take paclitaxel and cisplatin combined with angiogenesis blockade bevacizumab. However, the patient was afraid of the high price of bevacizumab and did not want to take chemotherapy which may have the side effect of hair loss. She asked for a relatively moderate and inexpensive treatment. Thus, pemetrexed 500 mg/m2 plus cisplatin 50 mg/m2 on day 1, cycled every three weeks for four cycles were performed to the patient. The RECIST 1.1 standard was used to evaluate the efficacy of the treatment. After two cycles, the MRI showed the patient’s pancreatic tail, abdominal, pelvic, and vaginal stump cystic lesions had not changed (Figure 1E-H), but the tumor markers were declined (Figure 3 and Table 1). While after four cycles, the lesions had shrunk (Figure 1I-L), and the tumor markers were sharply declined to the baseline (Figure 3 and Table 1). Consequently, the efficacy was evaluated as Stable Disease (SD) after two cycles, Partial Response (PR) after four cycles.
Table 1: The data of tumor markers during the treatment.
Time |
CEA (ng/ml) |
CA125 (U/ml) |
CA199 (U/ml) |
HE4 (pmol/L) |
2017/5/23 |
65.13 |
500.30 |
70.16 |
351.20 |
2017/6/26 |
35.30 |
217.10 |
81.95 |
103.30 |
2017/7/17 |
14.53 |
45.90 |
12.44 |
61.55 |
2017/8/8 |
9.64 |
20.40 |
8.10 |
55.23 |
2017/8/30 |
5.94 |
12.80 |
5.51 |
52.98 |
2017/9/24 |
3.78 |
5.60 |
8.86 |
49.89 |
2017/10/22 |
3.15 |
5.70 |
12.80 |
52.07 |
2017/11/15 |
2.39 |
5.10 |
9.83 |
52.94 |
2018/3/26 |
2.11 |
4.90 |
3.00 |
48.91 |
2018/5/30 |
3.02 |
4.80 |
2.80 |
45.80 |
2019/1/11 |
3.24 |
21.50 |
3.20 |
46.10 |
Abbreviations: CEA: Carcino-Embyrionic Antigen; CA125: Glycoprotein Antigen CA125; CA199: Glycoprotein Antigen CA199; HE4: Human Epididymis Protein 4.
To enhance the efficacy of the treatment, the doctor added anti-angiogenic drugs (apatinib) 500 mg/d to the pemetrexed and cisplatin for two cycles on September 2 and September 27. After the therapy, abdominal pelvic MRI showed that the patient’s abdominal cystic lesions were significantly reduced, but the pancreatic tail and pelvic cystic lesions had not changed to the previous ones. Thus, the efficacy was evaluated as PR (Figure 1M-P). Then the patient was continued to take oral apatinib treatment combined with single-agent pemetrexed to maintain chemotherapy for every 21 days from October 25 to November 16. As shown in (Figure 1Q-T), the lesions were not changed after these two cycles. After these, the patient was treated with single apatinib for maintenance therapy at home and went back to the hospital to evaluate the efficacy and adverse reaction every 3 to 6 months. As shown in Figure 1 U-X, in February 2019, the MRI showed the cystic lesions in the pancreatic tail, abdominal were disappeared. While for the part in the pelvic and the vaginal stump, the cystic lesions were sharply shrunk but still exist. The tumor markers remained normal (Figure 3 and Table 1). Now, the patient is continued taking apatinib as maintenance therapy. The progression-free survival time is more than twenty months.
The patient experienced no III-IV adverse events. All the toxicities were controllable and tolerable. According to the NCI-CTC for Adverse Events 5.0, the toxicities were described as follows: Diarrhea (grade 2), hand-foot syndrome (grade 2), hypertension (grade 1), the decline of platelet (grade 1) and leukocyte (grade 2), and fatigue (grade 1).
Discussion
Currently, recurrent and metastatic cervical cancer that cannot be resected or treated with radiotherapy is often incurable due to the lack of effective treatment. In this case, it is interesting to find that the patient with recurrent and metastatic cervical cancer achieves PFS of 21 months after treating with apatinib combined with pemetrexed-cisplatin. The toxicity can be tolerated, and no grade III-IV adverse effects have been found.
Generally speaking, the 5 years survival rate for the patients with cervical cancer in stage IA and IB (without high-risk factors and middle-risk factors) is nearly 85%-95% [4].However, the disease-free survival time of this patient was no more than 2 years. It might be related to the significantly lower survival in the patients with Adenocarcinoma (AC) than in Squamous Cervical Carcinoma (SCC), which has been reported by some researchers [19,20]. Nevertheless, some other researchers have reported that the histological type is not the determinant factor to affect prognosis [21]. No difference in survival outcomes between AC and SCC was found in the patients who did not require postoperative treatment [22]. More interestingly, in patients of stage IA and IB who have received postoperative treatment, the prognosis was relatively poorer in AC than in SCC [23,24]. May be the current postoperative treatments were not benefited people in the AC subtype. Individualized treatments should be studied in the future.
Pemetrexed is an antifolate preparation that contains a pyrrolopyrimidine-based nucleus and is widely used in lung adenocarcinoma and pleural mesothelioma [7,25]. It can inhibit cell replication by disrupting the folate-dependent metabolic processes [26]. Thymidylate Synthase (TS) is one of the targeted multiple enzymes by pemetrexed [27]. Increased the expression of TS could lead to radioresistant in cervical cancer cells and induce a relatively poor prognosis in patients with cervical cancer [28,29]. Based on these biological rational and the efficacy in other cancers, pemetrexed has been tested in four phase II trials among patients with advanced, persistent, metastatic, or recurrent cervical cancer (Table 1) [6,30]. In this case, the patient achieved a PR after treating with pemetrexed plus cisplatin as first-line chemotherapy for four cycles. As shown in Table 1, the GOG-0076GG series was designed to evaluate the safety and efficacy of pemetrexed combined with cisplatin as first-line chemotherapy in patients with advanced, persistent, or recurrent cervical cancer [6]. The overall response rate, the median PFS, and OS was 31% (one complete and 16 partial), 5.7 months, and 12.3 months, respectively [6]. The more grade 3 and 4 toxicities were neutropenia 35%, leukopenia 28%, and metabolic 28% [6]. Notably, among the patients with measurable tumors, the response rate was 38% of the patients in nonradiated disease sites, whereas 0% of that in irradiated disease sites [6]. So it might elucidate that the efficacy of pemetrexed-cisplatin is higher in the patients with metastatic and recurrent disease outside the irradiated sites than in the irradiated sites. In this case, most of the lesions were outside the irradiated sites. To some extent, this might be one of the reasons why the patient had a good effect. Moreover, on the basis of GOG-0204 which was designed to compare four cisplatin-based chemotherapy in stage IVB, recurrent, or persistent cervical cancer, the group of cisplatin plus paclitaxel were superior to other cisplatin doublets (topotecan, gemcitabine, and vinorelbine combined with cisplatin) [31]. The response rate, PFS, and OS of this group were 29%, 6 months, and 13 months, respectively [31]. And the more serious toxicities (grades 3 and 4) included neutropenia 78%, leukopenia 63%, and other hematologic 36% [31]. It seems that the cisplatin-pemetrexed was not inferior to cisplatin-paclitaxel with similar survival but lower toxicities. This series and this case report indicated that pemetrexed plus cisplatin might be effective cisplatin doublets in cervical adenocarcinoma, which could be a substitute for paclitaxel and cisplatin and need to be further explored in the future.
The expression of Vascular Endothelial Growth Factor (VEGF) and Hypoxia-Inducible Factor 1α (HIF-1α) is increased in high-grade cervical dysplasia and cervical cancer [32]. The HPV oncoproteins E6 and E7 could promote the growth of tumor neovascularization by enhancing both HIF-1α and VEGF expression [32]. Some studies have revealed that tumor neovascularization is related to poor prognosis in cervical cancer [33,34]. Thus, the small molecular antiangiogenesis drug bevacizumab has been added to regimens to investigate whether the bevacizumab could extend the response rate, PFS, and OS in patients with advanced or recurrent cervical cancer. As reported by GOG-0240, combined bevacizumab with cisplatin plus paclitaxel significantly increased the response rate, PFS, and OS, from 36%, 5.9 months, 13.3 months, to 48%, 8.2 months, 17months, respectively [35]. Data from ovarian cancer [8] and lung cancer [7] suggested that bevacizumab could also be added to pemetrexed plus cisplatin.
However, the bevacizumab is not suitable for Chinese people for its high price and inconvenience. A new small molecular antiangiogenesis drug-apatinib, was produced to benefit Chinese people. It was approved by China State Food and Drug Administration (CSFDA) as a treatment for gastric cancer patients after the second-line treatment. Recently, many studies have reported the efficacy of apatinib on lung cancer [36], liver cancer [37], breast cancer [38], esophageal cancer [39], pancreatic cancer [40], and so on. In cervical cancer cells, apatinib could inhibit cell proliferation by increasing cell apoptosis and G1 phase arrest [10]. The expression of VEGFR2 in cervical cancer tissues is positively related to the sensitivity of apatinib [10]. In vivo and in vitro experiments showed apatinib enhanced the sensitivity to paclitaxel [10]. Based on these biological rationales, some studies of apatinib in patients with cervical cancer are ongoing (Table 2). Recently, some studies have shown that apatinib is an effective and well-tolerated drug for cervical cancer [12-18]. But till now, no result has been reported to reveal the clinical efficacy and safety of apatinib combined with pemetrexedcisplatin in patients with cervical cancer. In this case, we have reported a patient who was administrated apatinib combined with cisplatin plus pemetrexed and shown PR after the therapy. Then a single dose of apatinib as maintenance therapy has controlled the disease for 15 months. It seems that the apatinib combined with cisplatin plus pemetrexed has extended the PFS in patients with recurrent or metastatic cervical cancer. But due to the lack of high-quality clinical research on this area, the efficacy of pemetrexed-cisplatin combined with apatinib need to be further studied.
Table 2: Summary of pemetrexed in four phase II trials among patients with recurrent/advanced cervical cancer.
Author |
n |
ORR (%) |
mPFS |
mOS |
Goedhals et al. [30] |
35 |
18 |
Median duration of 4 months |
|
Miller et al. [41] |
29 |
15 |
3.1 months |
7.4 months |
Lorusso et al. [42] |
43 |
14 |
10 weeks |
35 weeks |
Miller et al. [6] |
54 |
31 |
5.7 months |
12.3 months |
Abbreviations: ORR: Overall Response Rate; mPFS: Median Progression Free Survival; mOS: Median Overall Survival.
Conclusion
In conclusion, apatinib combined with pemetrexed plus cisplatin might be an effective and tolerated treatment for patients with recurrent and metastatic cervical cancer. Further prospective clinical trials are needed to be designed to verify the role of pemetrexed-cisplatin combined with antiangiogenesis drug in cervical cancer. Phase III trials are required to be conducted to elucidate whether the pemetrexed plus cisplatin combined antiangiogenesis drugs is superior to paclitaxel plus cisplatin combined with antiangiogenesis or not.
Declarations
Acknowledgments: This case report was supported by National Natural Science Foundation of China (81703037), and Zhongnan Hospital of Wuhan University Science Technology and Innovation Seed Fund Project (cxpy20160008, znpy2019078, znpy2019080).
Disclosure: The authors report no conflicts of interest in this work.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71: 209-249.
- Goodman A. HPV testing as a screen for cervical cancer. BMJ. 2015; 350: h2372.
- Liu Y, Wu L, Tong R, Yang F, Yin L, et al. PD-1/PD-L1 Inhibitors in Cervical Cancer. Frontiers in pharmacology. 2019; 10: 65.
- Chen J, Gu W, Yang L, Chen C, Shao R, et al. Nanotechnology in the management of cervical cancer. Rev Med Virol. 2015; 25:72- 83.
- Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol. 2016; 214: 22-30.
- Miller DS, Blessing JA, Ramondetta LM, Pham HQ, Tewari KS, et al. Pemetrexed and cisplatin for the treatment of advanced, persistent, or recurrent carcinoma of the cervix: A limited access phase II trial of the gynecologic oncology group. J Clin Oncol. 2014; 32: 2744-2749.
- Zhan M, Zheng H, Xu T, Yang Y, Li Q. Cost-effectiveness analysis of additional bevacizumab to pemetrexed plus cisplatin for malignant pleural mesothelioma based on the MAPS trial. Lung Cancer. 2017; 110: 1-6.
- Hagemann AR, Novetsky AP, Zighelboim I, Gao F, Massad LS, et al. Phase II study of bevacizumab and pemetrexed for recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancer. Gynecol Oncol. 2013; 131: 535-540.
- Tian S, Quan H, Xie C, Guo H, Lu F, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer science. 2011; 102: 1374-1380.
- Qiu H, Li J, Liu Q, Tang M, Wang Y, et al. Apatinib, a novel tyrosine kinase inhibitor, suppresses tumor growth in cervical cancer and synergizes with Paclitaxel. Cell Cycle. 2018; 17: 1235-1244.
- Zhou JG, Zhou NJ, Zhang Q, Feng YY, Zhou H. Apatinib for patients with advanced or recurrent cervical cancer: Study protocol for an open-label randomized controlled trial. Trials. 2018; 19: 500.
- Chen C, Qin S, Li Z, Luo X, Zhang Y, et al. A retrospective sixpatient series of apatinib for the treatment of persistent or recurrent carcinoma of the cervix. OncoTargets and therapy. 2019; 12: 5805-5811.
- Yu J, Xu Z, Li A, Zhang J, Wang Y, Zhao H, et al. The Efficacy And Safety Of Apatinib Treatment For Patients With Metastatic Or Recurrent Cervical Cancer: A Retrospective Study. Drug Des Devel Ther. 2019; 13: 3419-3424.
- Su M, Gao Y, Ye X, Zhou Q, Zhao L, et al. Clinical Value Of Apatinib As A Salvage Treatment In Patients With Chemo-Refractory Advanced Cervical Cancer. OncoTargets and therapy. 2019; 12: 9707-9713.
- Li N, Wang Z, Yuan G, Sun Y, Zhang R, et al. An Oral Small Molecule VEGFR2 Inhibitor, Apatinib, in Patients with Recurrent or Refractory Cervical Cancer: A Real World Study. J Oncol. 2020; 2020: 3852373.
- Xiao Y, Cheng H, Wang L, Yu X. Clinical response and safety of apatinib monotherapy in recurrent, metastatic cervical cancer after failure of chemotherapy: A retrospective study. Journal of gynecologic oncology. 2020; 31: e2.
- Zhang L, Chen L, Yu H. Phase II study of apatinib, a novel tyrosine kinase inhibitor targeting tumor angiogenesis, as second-line treatment for recurrent or advanced cervical cancer patients. Investigational new drugs. 2020; 38: 1186-1191.
- Yang H, Chen M, Mei Z, Xie C, Zhou Y, et al. Effectiveness and prognostic factors of apatinib treatment in patients with recurrent or advanced cervical carcinoma: A retrospective study. Cancer medicine. 2021; 10: 4282-4290.
- Zhou J, Wu SG, Sun JY, Li FY, Lin HX, et al. Comparison of clinical outcomes of squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma of the uterine cervix after definitive radiotherapy: A population-based analysis. Journal of cancer research and clinical oncology. 2017; 143: 115-122.
- Lee JY, Kim YT, Kim S, Lee B, Lim MC, et al. Prognosis of Cervical Cancer in the Era of Concurrent Chemoradiation from National Database in Korea: A Comparison between Squamous Cell Carcinoma and Adenocarcinoma. PLoS One. 2015; 10: e0144887.
- Katanyoo K, Sanguanrungsirikul S, Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol. 2012; 125: 292-296.
- Yamauchi M, Fukuda T, Wada T, Kawanishi M, Imai K, et al. Comparison of outcomes between squamous cell carcinoma and adenocarcinoma in patients with surgically treated stage I-II cervical cancer. Molecular and clinical oncology. 2014; 2: 518-524.
- Yanaranop M, Tuipae S, Nakrangsee S. Comparison of Survival Outcomes in Early Stage Invasive Adenocarcinoma with Squamous Cell Carcinoma of the Uterine Cervix. J Med Assoc Thai. 2017; 100: S77-S86.
- Noh JM, Park W, Kim YS, Kim JY, Kim HJ, et al. Comparison of clinical outcomes of adenocarcinoma and adenosquamous carcinoma in uterine cervical cancer patients receiving surgical resection followed by radiotherapy: A multicenter retrospective study (KROG 13-10). Gynecol Oncol. 2014; 132: 618-623.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003; 21: 2636-2644.
- Zhao R, Babani S, Gao F, Liu L, Goldman ID. The mechanism of transport of the Multitargeted Antifolate (MTA) and its crossresistance pattern in cells with markedly impaired transport of methotrexate. Clinical cancer research : An official journal of the American Association for Cancer Research. 2000; 6: 3687-3695.
- Wang Y, Zhao R, Goldman ID. Characterization of a folate transporter in HeLa cells with a low pH optimum and high affinity for pemetrexed distinct from the reduced folate carrier. Clinical cancer research: An official journal of the American Association for Cancer Research. 2004; 10: 6256-6264.
- Manegold C, Symanowski J, Gatzemeier U, Reck M, von Pawel J, Kortsik C, et al. Second-line (post-study) chemotherapy received by patients treated in the phase III trial of pemetrexed plus cisplatin versus cisplatin alone in malignant pleural mesothelioma. Ann Oncol. 2005; 16: 923-927.
- Bonomi P, Blessing JA, Stehman FB, DiSaia PJ, Walton L, et al. Randomized trial of three cisplatin dose schedules in squamouscell carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol. 1985; 3: 1079-1085.
- Goedhals L, van Wiyk AL, Smith BL, Fourie SJ. Pemetrexed (Alimta, LY231514) demonstrates clinical activity in chemonaive patients with cervical cancer in a phase II single-agent trial. International journal of gynecological cancer: Official journal of the International Gynecological Cancer Society. 2006; 16: 1172- 1178.
- Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 2009; 27: 4649- 4655.
- Tang X, Zhang Q, Nishitani J, Brown J, Shi S, et al. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clinical cancer research : An official journal of the American Association for Cancer Research. 2007; 13: 2568- 2576.
- Fujimoto J, Toyoki H, Sato E, Sakaguchi H, Jahan I, et al. Expression of cyclooxygenase-2 related to angiogenesis in uterine cervical cancers. J Biomed Sci. 2006; 13: 825-832.
- Lara PC, Lloret M, Clavo B, Apolinario RM, Henriquez Hernandez LA, et al. Severe hypoxia induces chemo-resistance in clinical cervical tumors through MVP over-expression. Radiat Oncol. 2009; 4: 29.
- Tewari KS, Sill MW, Long HJ, 3rd Penson RT, Huang H, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014; 370: 734-743.
- Luo H, Zhang L, Yang B, Feng Y, Xiong Y, et al. A randomized phase 2 trial of apatinib vs observation as maintenance treatment following first-line induction chemotherapy in extensive- stage small cell lung cancer. Investigational new drugs. 2020; 38: 148- 159.
- . Zhen L, Jiali C, Yong F, Han X, Hongming P, Weidong H, et al. The Efficacy and Safety of Apatinib Treatment for Patients with Unresectable or Relapsed Liver Cancer: A retrospective study. J Cancer. 2018; 9: 2773-2777.
- Zhu A, Yuan P, Wang J, Fan Y, Luo Y, Cai R, et al. Apatinib combined with chemotherapy in patients with previously treated advanced breast cancer: An observational study. Oncology letters. 2019; 17: 4768-4778.
- Li J, Jia Y, Gao Y, Chang Z, Han H, et al. Clinical efficacy and survival analysis of apatinib combined with docetaxel in advanced esophageal cancer. OncoTargets and therapy. 2019; 12: 2577- 2583.
- Liang L, Wang L, Zhu P, Xia Y, Qiao Y, et al. Apatinib concurrent gemcitabine for controlling malignant ascites in advanced pancreatic cancer patient: A case report. Medicine. 2017; 96: e8725.
- Miller DS, Blessing JA, Bodurka DC, Bonebrake AJ, Schorge JO. Gynecologic Oncology G. Evaluation of pemetrexed (Alimta, LY231514) as second line chemotherapy in persistent or recurrent carcinoma of the cervix: A phase II study of the Gynecologic Oncology Group. Gynecologic oncology. 2008; 110: 65-70.
- Lorusso D, Ferrandina G, Pignata S, et al: Evaluation of pemetrexed (Alimta, LY231514) as second-line chemotherapy in persistent or recurrent carcinoma of the cervix: The CERVIX 1 study of the MITO (Multicentre Italian Trials in Ovarian Cancer and Gynecologic Malignancies) Group. Annals of oncology : Official journal of the European Society for Medical Oncology. 2010; 21: 61-66.