Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Short Report - Open Access, Volume 5
Management of foldable capsular vitreous body implant exposure: First reported case
Catriona J Downie1,2,3*; Christopher Qureshi1; Krishna Tumuluri1,2,3
1Department of Ophthalmology, Westmead Hospital, Westmead, New South Wales, Australia.
2Department of Ophthalmology, Sydney Eye Hospital, Sydney, New South Wales, Australia.
3Faculty of Medicine and Health, Save Sight Institute, Sydney Medical School, The University of Sydney, Australia.
*Corresponding Author : Catriona J Downie
Department of Ophthalmology, Westmead Hospital,
Westmead, New South Wales, Australia.
Email: catriona.downie@gmail.com
Received : Nov 13, 2024
Accepted : Nov 29, 2024
Published : Dec 06, 2024
Archived : www.jcimcr.org
Copyright : © Downie CJ (2024).
Abstract
Management of phthisis bulbi is challenging for ophthalmologists, and often results in evisceration. Foldable capsular vitreous bodies have been developed as an alternative to evisceration in eyes with chronic retinal detachment. We present a case of foldable capsular vitreous body implant extrusion, which necessitated removal and evisceration. This case highlights risks associated with their use, and the importance of appropriate patient selection. Further study and investigation are required to confirm the long-term safety of foldable capsular vitreous body implants.
Keywords: Foldable capsular vitreous body; Phthisis; Extrusion; Phthisis bulbi.
Abbreviations: FCVB: Foldable Capsular Vitreous Body; SO: Silicone Oil.
Citation: Downie CJ, Qureshi C, Tumuluri K. Management of foldable capsular vitreous body implant exposure: First reported case. J Clin Images Med Case Rep. 2024; 5(12): 3376.
Introduction
Management of phthis bulbi has been limited to supportive measures, evisceration and enucleation. The foldable capsular vitreous body (FCVB - Vesber, Guangzhou, Guandong, China) [1] is a new intraocular implant to address phthisis. It replaces vitreous cavity volume while avoiding an interface between oil and the eye. It aims to prevent progression of phthisis. However, little is published regarding complications associated with its use. As it becomes more prevalent, it is important ophthalmologists are aware of potential complications and their management.
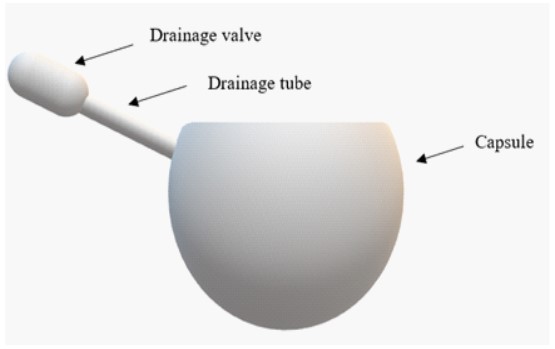
The FCVB (Figure 1) is made of medical grade silicone rubber [2]. It is inserted into the eye following standard three port pars plana vitrectomy. The valve is positioned under the conjunctiva, and the conjunctival incision is closed with absorbable sutures.
Case presentation
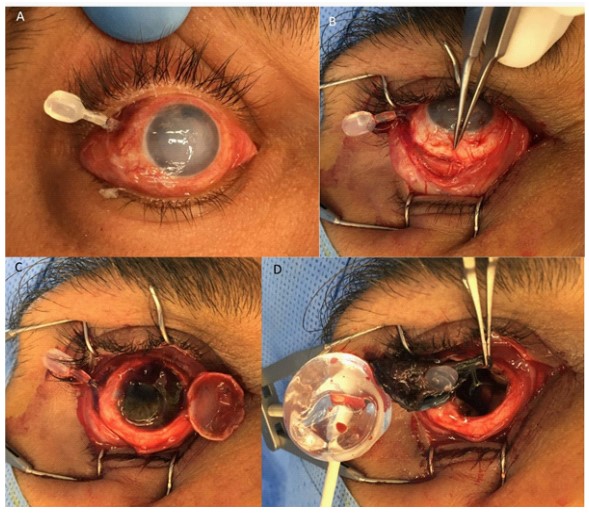
A FCVB was implanted to the right eye of a 38 year old man following recurrent retinal detachment due to penetrating eye injury. He had previously undergone two pars plana vitrectomies with Silicone Oil (SO) insertion and placement of an encircling band. Four months after the FCVB insertion he was found to have extrusion of the valve, and underwent evisceration and removal of the FCVB (Figure 2).
Discussion
There are few published reports regarding FCVBs. The data collected are heterogenous so are unsuitable for quantitative comparison. All eyes have either complex retinal detachments or extensive posterior segment injury requiring long term SO use. Most patients were over 18 years of age, but some series included children [1,3,4]. The duration of follow up was variable, but mostly under 12 months. One study documented a case of extruded drainage valve [5], but did not comment on its management. To our knowledge, this is the first published case report discussing the management of an extruded FCVB. The use of intraocular implants in patients with poor or no visual potential represents a shift from the paradigm of avoiding surgery due to the risk of developing sympathetic ophthalmia in the fellow eye [6]. This requires careful preoperative discussion with the patient so they are aware of the risks of surgery. The decision to proceed is balanced by the consideration of the physical and psychological impacts of removing the phthisic eye.
We proceeded with evisceration as the eye had no visual potential and had already undergone multiple surgeries. It was felt that repositioning the valve would likely lead to further extrusion due to mechanical erosion with the scleral buckle. Few reported cases involved concurrent scleral buckle. Liu et al. [7] report two of their five patients had a scleral buckle and no extrusion was documented during their 12 month follow up period. The encircling band is a possible cause of drainage valve ex- trusion in our patient, as it made it difficult to position the valve in its normal subconjunctival location, and resulted in the valve sitting more anteriorly. This may have resulted in increased mechanical irritation between the valve and the overlying conjunctiva, leading to its erosion. This case highlights risks associated with a new implant to address phthisis. Ophthalmologists should be aware of this device and its possible wider use. Further research is needed to examine the indications, long-term outcomes and complications with FCVB implants.
References
- Lin X, Ge J, Gao Q, et al. Evaluation of the flexibility, efficacy, and safety of a foldable capsular vitreous body in the treatment of severe retinal detachment. Investigative ophthalmology & visual science. 2011; 52(1): 374-381.
- Zeng B, Wang Q, Sui G, Wang M, Xie W, et al. Foldable capsular vitreous body implantation for treatment of traumatic retinal detachment: two case reports. Journal of International Medical Research. 2021; 49(2): 0300060521990257.
- Zhang R, Wang T, Xie C, et al. Evaluation of supporting role of a foldable capsular vitreous body with magnetic resonance imaging in the treatment of severe retinal detachment in human eyes. Eye. 2011; 25(6): 794-802.
- Zhang X, Tian X, Zhang B, Guo L, Li X, et al. Study on the effectiveness and safety of Foldable Capsular Vitreous Body implantation. BMC Ophthalmology. 2019; 19(1): 260. doi:10.1186/ s12886-019-1268-x.
- Chen S, Tian M, Zhang L, et al. Reattachment After Foldable Capsular Vitreous Body Implantation in Severe Retinal Detachment Eyes. Translational Vision Science & Technology. 2021; 10(11): 8-8.
- Parchand S, Agrawal D, Ayyadurai N, et al. Sympathetic ophthalmia: A comprehensive update. Indian Journal of Ophthalmology. 2022; 70(6): 1931.
- Liu N, Kang L, Yu X, et al. Preliminary clinical application of foldable capsular vitreous body in severe silicone oil-de- pendent eyes. Ann Palliat Med. 2021; 10(10): 10922-10929. doi:10.21037/apm-21-2554.