Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
Evaluation of the anti-diabetic activities of crude extracts of Annona muricata and Rutidea parviflora in alloxan-diabetic rats
OR Johnson-Ajinwo*; Kuebari Penuel Berebari
Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Nigeria.
*Corresponding Author : OR Johnson-Ajinwo
Department of Pharmaceutical and Medicinal
Chemistry, Faculty of Pharmaceutical Sciences,
University of Port Harcourt, Nigeria.
Email: okiemute_2002@yahoo.co.uk
Received : Mar 05, 2021
Accepted : Apr 16, 2021
Published : Apr 21, 2021
Archived : www.jcimcr.org
Copyright : © Johnson-Ajinwo OR (2021).
Abstract
Background: Diabetes Mellitus (DM), is a malignant disease that has impacted the globe in astronomical proportions. Chronic hyperglycemia is a major condition suffered by patients with DM. Currently, the cost of managing DM is highly exorbitant and poses a significant obstacle to many living with the disease in developing countries. Thus, the need for affordable alternatives with optimum potency and minimal side effects is justified. Historically, Annona muricata is one plant that is used in the treatment of DM and a host of other ailments. Rutidea parviflora has been used in combination with other plants in the treatment of DM by some ethnic groups. The aim of this study is to investigate the anti-diabetic activities of these plants.
Method: The anti-diabetic activities of aqueous and organic extracts of Annona muricata (leaves), and Rutidea parviflora (root bark) at 200 mg/kg and 400 mg/kg, were screened for their anti-diabetic activities in normoglycemic and alloxan-induced diabetic rats (150-200 g) at 1h, 2h, 4h, 6h, 8h, and the 12th hour. DMSO (0.3 ml of 33.3% v/v stock) and Glibenclamide 10 mg/kg were administered to the control and reference groups respectively.
Result: Organic extracts of A. muricata at 400 mg/kg significantly decreased FBGL in both normoglycemic and hyperglycemic groups by 65.9% and 62.1% respectively (P<0.05). R. parviflora leaves extracts showed less activity. However, it had relatively short acting activity (maximum activity at the 4th hour). In each case, the organic extracts performed better than the aqueous extracts.
Conclusion: A. muricata has potentials for the treatment of DM, and merits further research to support the plant’s therapeutic application. R. parviflora may offer some beneficial effect, and possibly boost the potency of A. muricata by synergistic activity when co-administered.
Keywords: Rutidea parviflora; Annona muricata ; hyperglycemia.
Citation: Johnson-Ajinwo OR, Berebari KP. Evaluation of the anti-diabetic activities of crude extracts of annona muricata and rutidea parviflora in alloxan-diabetic rats. J Clin Images Med Case Rep. 2021; 2(2): 1056.
Introduction
Diabetes Mellitus (DM) is a debilitating disorder characterized by severe hyperglycemia owing to insufficient insulin production. The disease is a major contributor to other devastating health conditions which include; kidney failure, stroke, cardiac arrest, blindness and could lead to amputation of lower limbs in complicated cases. In three and a half decades, the number of people living with DM rose from 108 million to 422 million, [1] with a further projection that by 2045, there would be 629 million adults living with DM [2]. As at 2012, the death toll from DM was 1.6 million, which paints a gloomy picture of an enhancing disease with lethal consequences. The economic burden imposed by the disease was 1.31 trillion US dollars in 2015 [3]. The challenge of managing DM is heaviest on developing countries, where affordable healthcare is beyond the reach of many. DM is ranked the seventh killer disease globally and an expensive disease to manage. Thus, the drive for cheaper and more potent therapies; mainly herbal remedies cannot be overemphasized.
A. muricata is a tropical shrub, commonly known as soursop or graviola. The fruits of A. muricata are highly coveted for their unique flavor and nutritional value. A wide array of ethnomedicinal activities has been attributed to various parts of A. muricata. Ethnopharmacological uses of A. muricata abound in African and South American communities. Many studies has been carried out on a number of biological activities such as anti-diabetic, anti-cancer, anti-convulsant, anti-parasitic and antiarthritic, as seen from the review of this plant [4]. Annonaceous acetogenins were found to be the most abundant constituents isolated from the different parts of the plant [4].
There have been documented cytotoxic activities of A. muricata against several human cell lines and disease agents in in vitro culture and in vivo models designed to specifically target the disease, while exerting little or no effect on normal cell viability [5]. It has been reported also that annonaceous acetogenins found only in the Annonaceae family kill malignant cells of 12 different types of cancer including Breast, Ovarian, Colon, Prostate, Liver, Lung, Pancreatic and Lymphoma [6].
Rutidea parviflora D.C belongs to the genus Rutidea in the Rubiaceae family. Rubiaceae referred to as the coffee family or bedstraw family is the fourth largest family of the angiosperms. Rubiaceae has 611 genera and approximately 13500 species distributed in different regions of the world, but with greater diversing in the sub-tropical areas. Two important genera of this family are Cinchona L., from which the drug quinine used in the treatment of malaria was derived and Coffea L., which have provided the world with the most consumed beverage-coffee. Other genera are Rubia, a collection of dye plants and used for decorative purposes, Pavetta L., Uncaria schreb, Tarenna Gaertn, Oldenlandia L., and Galium L [7]. This plant is used by the indigenous people of Ethiope East and West Local Government in Delta State, Nigeria for inflammation, infections and a host of other disease conditions, which has necessitated this research to investigate the ethno pharmacological use of this plant for anti-diabetic activities. This plant has scanty reports in literature and has also shown some anti-cancer activity in vitro.
Materials and methods
I. Plant materials and reagents
The leaves of A. muricata were sourced from household gardens around the University community, while the root bark of R. parviflora were obtained from a bio reserve. The plant materials were air-dried under shade before being milled into fine particles. The following reagents were utilized in the extraction and phytochemical work: Methanol, Dichloromethane, Chloroform, Diethyl Ether, Acetic Anhydride, Glacial acetic acid, Sodium picrate, 2% 3,5-Dinitrobenzoic acid, Picric acid, Iodine solution, Dimethylsulfoxide, (JHD company, Guangdong. Guanghua Sci-Tech. Co. Ltd. China), Hydrochloric acid, Million’s reagent, Benedict’s solution, Wagner’s reagent, Sodium Hydroxide, Ferric chloride solution, Saturated lead acetate solution, Dragendorff’s reagent, Kedde reagent, Ammonia solution, 7.5% Potassium Hydroxide, Fehling’s solution A and B (Sigma Aldrich Chemicals, St Louis, USA), Distilled water, Deionized water (Pharmaceutical Chemistry Lab, University of Port Harcourt).
II. Assay materials
Alloxan monohydrate (Sigma-Aldrich Merck Germany), Glibenclamide (Actiza Pharmaceutical Private Limited, Surat), ACCU-CHECK glucometer.
III. Extraction of plant materials
The 2 Kg of the pulverized plant materials were extracted according to the American National Cancer Institute (NCI) method of extraction [8]. The pulverized plant material was macerated in a 1:1 mixture of dichloromethane and methanol for 24 h to obtain the extracts. The procedure was repeated thrice. The ratio of pulverized plant material to extraction solvent was 1:5. The residue was further macerated in methanol for another 24 h to yield the methanol extract, which was combined with the dichloromethane/methanol extract to yield the total organic extract. The obtained dry extracts were further dried in a desiccator to remove any trace of solvent. The residue was later macerated in deionized water for 24 hour to yield the aqueous extract, which was dried on a lypholizer. Both organic and aqueous extracts were obtained for both plants.
IV. Phytochemical screening
Phytochemical tests were carried out on the plants’ extract by the method described by Trease and Evans, [9] highlighted below.
i. Test for flavonoids
a. Shinoda reduction test
100 mg of the plant extract was dissolved in 5 ml of hydrochloric acid, a few pieces of magnesium metal was added to 5 ml of each plant extract solution. The presence of flavonoids is indicated by a reddish crimson or orange colour.
b. Sodium hydroxide test for flavonoids
A small amount of each of the portions was dissolved in water and filtered; to this, 2 ml of 10% aqueous sodium hydroxide was added to produce a yellow colouration. The addition of dilute hydrochloric acid, effected a colour change from yellow to colourless; indicative of the presence of flavonoids.
ii. Test for tannins
a. Ferric chloride test
100 mg of plant extract was boiled with 5 ml of distilled water for 5 minutes, cooled and filtered, few drops of 5% ferric chloride reagent was added. An observed green to blue black precipitate signified the presence of tannins.
iii. Test for alkaloids
5 ml of 2 M HCl was added to 0.1 g of plant extracts and heated on the steam bath for 2 minutes, the mixture was filtered. 1 ml of the filtered mixture was treated with 2 drops of the following reagents respectively: (i) Dragendorff’s reagent; a reddish precipitate signified the presence of alkaloid. (ii) Wagner’s reagent; yellow precipitate suggestive of the presence of alkaloid.
iv. Test for saponins
a. Frothing test
100 mg of the plant extract was mixed with 5 ml of water and shaken vigorously for persistent foam, then warmed and observed persistent foaming on warming indicated the presence of saponins.
b. Emulsion test
100 mg of plant extract dissolved in 10 ml of water was mixed with 3 drops of olive oil. The mixture was shaken vigorously. The formation of emulsion signifies a positive test for saponins.
v. Test for phlobatannins
Hydrochloride Acid Test 100 mg of plant extract was dissolved in water and filtered. The filtrate was boiled with 1% hydrochloric acid. Deposition of a red coloured precipitate indicates a positive test
vi. Test for sterols/triterpenoids
a. Liebermann-buchard test
100 mg of plant extract is dissolved in chloroform and acetic anhydride is added followed by sulphuric acid. Green colour indicates the presence of steroids and pink colour indicates terpenoids.
b. Salkwoski’s test
100 mg of plant extract was dissolved in 2 ml chloroform, then conc. H2 SO4 was carefully added to form a layer. An observed reddish brown colour at the interface strongly indicates a steroidal nucleus.
vii. Test for Cardiac glycosides
a. Keller killiani test
100 mg of plant extract was dissolved in about 5 ml of water, followed by the addition of glacial acetic acid, one drop of 5% FeCl3 and conc. H2 SO3 . Reddish brown colour that appears at the junction of the two liquid layers and an upper layer that appears to be bluish-green confirms the presence of glycosides.
b. Kedde test
About 5 ml of dissolved extract was treated with a small amount of Kedde reagent (which was prepared by combining the same volumes of 2% solution of 3, 5 dinitrobenzoic acid in menthol to a 7.5% aqueous solution of KOH). The formation of a blue or violet colour that fades out in 1-2 hours indicates the presence of cardiac glycoside.
viii Test for carbohydrates
a. Fehling’s test
A few drops of Fehling’s solution A + B was added to 0.1 g of the plant extracts and boiled for a few minutes. The presence of deep blue to green; yellow to red colour change indicates a positive reduction test.
b. Molish’s test
100 mg of plant extract was dispersed in 5 ml of distilled water and the resulting mixture allowed to boil for 15 minutes and filtered. Molish reagent (1 ml) was added to the filtrate (1 ml) and agitated, followed by addition of 1 ml of concentrated H2 SO4 . A reddish ring was observed indicative of carbohydrate.
ix. Test for proteins
About 100 mg of the sample was extracted with distilled water by boiling and subsequent filtration.
a. Million’s test
To a little portion of the filtrate in a test tube, 2 drops of Million’s reagent was added. Formation of white Precipitate which indicates the presence of protein was watched out for.
b. Picric acid test
To a little portion of the filtrate were added drops of Picric acid solution. A yellow Precipitate indicates the presence of protein.
x. Tests for fixed oils and fats
Oil Stain test: The extracts were placed between two filter papers and pressure exerted on the filter papers. The detection of oil stains on the papers indicated the presence of fixed oils.
V. Experimental design
This study was designed in line with the ethically approved experimental protocols adopted by the department of Experimental Pharmacology and Toxicology, of the Faculty of Pharmaceutical Sciences, University of Port Harcourt. Adult mixed-gender wistar rats weighing between 150-200 g, bred by the animal house unit of the department of Experimental Pharmacology and Toxicology were used for the study. The rats were housed in spacious cages, to allow for free movement at room temperature, sufficient humidity and 12 hourly cycles of light and darkness. The animals, had access to standard laboratory animal feed and water prior to the commencement of the experiment.
The rats were randomly divided into 14 groups of 6 rats each: 8 test groups and 6 control groups and were fasted overnight. The 14 groups were sub-divided into two categories, A and B. Category A consist of normoglycemic rats that received the extracts and the controls. While category B were alloxan-induced rats that were treated with the extracts and the controls respectively
Freshly prepared alloxan solution (in 0.9% w/v normal saline) was administered at a dose of 150 mg/kg Intraperitoneally to normoglycemic rats. Those with FBGL>200 mg/dL after 72hrs were considered to have developed experimental diabetes. The FBGL of the animals were determined to ensure that they were not diabetic before carrying out the actual experiment. ACCUCHECK glucometer was used in BGL determination. The blood analyzed was gotten by pricking the tail of the animals.
4 of the 14 test groups were treated with intraperitoneal injection of organic extract, one dose per group (200 mg/kg and 400 mg/kg) respectively. Another 4 group received intraperitoneal injection of aqueous extract, one dose per group (200 mg/kg and 400 mg/kg) respectively. The 6 control groups, were treated as follows: One was untreated, 0.3 ml of normal saline was administered to 1 group; two other groups were treated with I. P. injection of 0.3 ml of the DMSO stock while the last two group were treated with Glibenclamide 10 mg/kg (I.P.)
The Maximum Reduction was calculated using the formula:
(Initial reading after Induction) – (Lowest reading obtained after treatment) While the percentage Maximum Reduction was calculated using the formula:
VI. Statistical analysis
The analytical tool used was Graph pad Prism version 8 for calculation of the mean values ± Standard Error of Mean (SEM). The one-way ANOVA was used to determine statistical difference between means. A p<0.05 was considered statistically significant.
Results
Results from the phytochemical screening
Table 1 displays the result for the phytochemical screening of the aqueous and organic extracts of the plants. The results showed that the organic extracts contained most of the plants constituents than the aqueous extracts. Previous studies on A. muricata showed that methanol and water were the most used solvents of extraction, resulting in higher yield of phenolic compounds [10,11]. This may suggests the reason for the organic extracts giving more potent anti-diabetic activities than the aqueous extracts. Organic extracts of A. muricata also showed heavy Presence of lipids and moderate presence of carbohydrates, flavonoids, tannins alkaloids, phlobatannins and proteins while that of R. parviflora showed heavy Presence of alkaloids. In all the plant extracts having moderate to heavy Presence of tannins and flavonoids and alkaloids showed relatively high antidiabetic activities. This is affirmed by several research works including those by Yogesha Mohan et al. and Kossouoh C, et al [12,1].
Table 1: The results of the qualitative phytochemical tests of extracts of the plant materials
Test |
|
R. parviflora Extracts |
||
|
Aqueous |
Organic |
Aqueous |
Organic |
Flavonoids |
++ |
+ |
+ |
++ |
Tannins |
+ |
++ |
+ |
++ |
Alkaloids |
+ |
+++ |
Trace |
+++ |
Saponins |
++ |
++ |
++ |
+ |
Phlobatannins |
Trace |
++ |
- |
- |
Sterols/Terpenoids |
_ |
+ |
Trace |
+ |
Cardiac Glycosides |
+ |
++ |
+ |
++ |
Carbohydrates |
+ |
+ |
+ |
+ |
Proteins |
+ |
+ |
+ |
+ |
Fats and oils |
Trace |
++ |
Trace |
++ |
KEY: -: Absent; +: Slightly present; ++: Moderately present; +++: Heavily present.
Effect of Annona muricata leaves aqueous extracts on blood glucose levels (mg/kg) in normoglycemic and alloxan-treated diabetic rats.
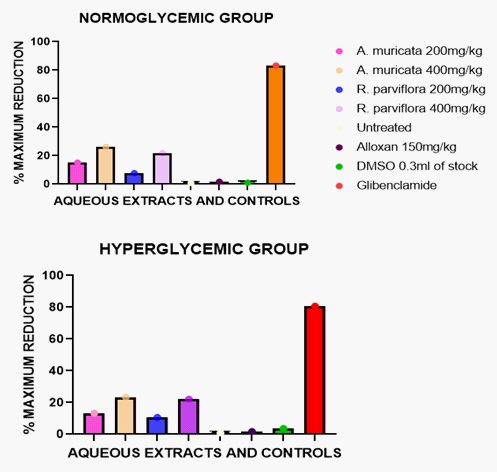
Tables 2.1 and 2.2 show the effect of aqueous extracts of A. muricata on FBGL+- SEM in normoglycemic and hyperglycemic rats respectively. The anti-diabetic effects of the aqueous extract dosed at 200 mg/kg and 400 mg/kg separately, were less potent, giving (15.2% and 26.2%) and (13.3% and 23.4%), in normoglycemic and hyperglycemic groups respectively, 12 hour post administration. This can be explained by the low secondary metabolites' concentration of the aqueous extract and possibly by the high carbohydrate and protein-secondary metabolites ratio which may serve as diluent to the active anti-diabetic compounds.
Table 2.1: Effect of A. muricata aqueous extract on blood glucose levels (mg/kg) in normoglycemic rats
Treatment Groups |
Before Treatment |
After Treatment |
Maximal Reduction |
% Maximal Reduction |
||||||
0h |
1h |
2h |
4h |
6h |
8h |
12h |
||||
Untreated |
77.3 ± 5 |
77.7 ± 3 |
79.7 ± 5 |
83.3±5 |
91.6±6 |
91.7±4 |
76.7 ± 7 |
0.7 |
0.9 |
|
A. muricata |
||||||||||
200mg/kg |
74.7 ± 2 |
140.7 ± 5 |
131.7 ± 2 |
108.0 ± 1 |
96.3 ± 4 |
77.3 ± 5 |
63.3 ± 3 |
11.3 |
15.2 |
|
400mg/kg |
77.7 ± 6 |
86.0 ± 4 |
96.0 ± 4 |
91.3 ± 4 |
97.7 ± 6 |
84.7 ± 8 |
57.3 ± 1 |
20.3 |
26.2 |
|
DMSO (0.3ml |
65.7 ± 1 |
70.0 ± 2 |
70.0 ± 1 |
66.7 ± 2 |
68.3 ± 2 |
69.0 ± 1 |
65.0 ± 2 |
0.7 |
1.2 |
|
Glibenclamide (10mg/kg) |
79.0 ± 4 |
73.0 ± 4 |
63.3 ± 1 |
43.3 ± 4 |
31.3 ± 3* |
13.3 ± 1* |
Lo |
65.7 |
83.3 |
|
* = p<0.05 ≥ 200mg/dL FBGL = Diabetic. Lo = too low to be detected
Table 2.2: Effect of A. muricata aqueous extract on blood glucose levels (mg/kg) in diabetic rats.
Treatment Groups |
Before Treatment |
After Treatment |
Maximal Reduction |
% Maximal Reduction |
||||||
1h |
2h |
4h |
6h |
8h |
12h |
|||||
Untreated |
77.3 ± 5 |
77.7 ± 3 |
79.7 ± 5 |
83.3 ± 5 |
91.6 ± 6 |
91.7 ± 4 |
76.7 ± 7 |
0.7 |
0.9 |
|
Alloxan (150mg/kg) |
69.0 ± 6 |
475.5 ± 2 |
47.3 ± 2 |
514.8 ± 1 |
509.3 ± 8 |
495.3 ± 4 |
477.0 ± 1 |
467.1 ± 8 |
8.4 |
1.8 |
A. muricata |
||||||||||
200mg/kg |
80.0 ± 3 |
506.7.0 ± 7 |
547.8 ± 3 |
546.0 ± 2 |
579.8 ± 4 |
530.0 ± 5 |
482.8 ± 5 |
439.8 ± 4 |
73.0 |
13.3 |
400mg/kg |
82.0 ± 2 |
475.0 ± 7 |
494.3 ± 1 |
483.8±6 |
434.3 ± 4 |
383.0 ± 3 |
400.8 ± 3 |
382.3 ± 5 |
112.0 |
23.4 |
DMSO (0.3ml) |
76.3 ± 4 |
398.5 ± 6 |
400.5 ± 8 |
397.3±6 |
383.8 ± 1 |
398.3 ± 9 |
398.3 ± 7 |
401.0 ± 6 |
14.8 |
3.7 |
Glibenclamide (10mg/kg) |
85.5 ± 2 |
446.0 ± 2 |
311.8 ± 3 |
231.8±7 |
204.3 ± 7* |
135.3 ± 1* |
104.0 ± 3* |
86.0 ± 7* |
360.0 |
80.7 |
* = p<0.05 ≥ 200mg/dL FBGL = Diabetic
Effect of Annona muricata leaves organic extracts on blood glucose levels (mg/kg) in normoglycemic and alloxan-treated diabetic rats.
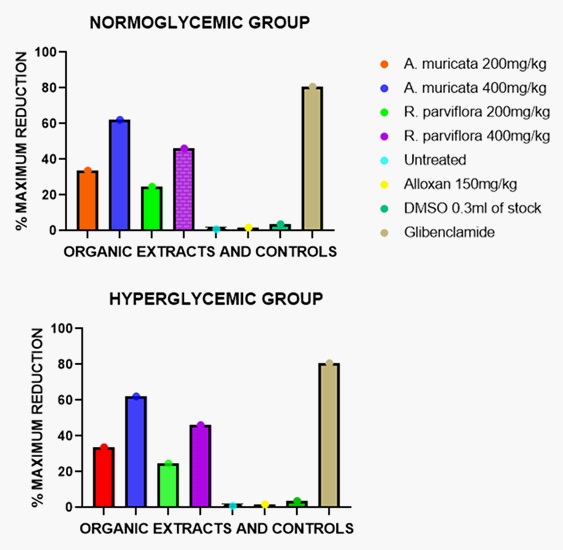
The results in Tables 3.1 and 3.2 show the anti-diabetic activity of the organic extracts of A. muricata in normoglycemic and hyperglycemic groups respectively. It was revealed that the organic extract has better potency than the aqueous extracts in a dose-dependent manner. In normoglycemic groups, the activity was found to be 35.3% and 65.9% while in hyperglycemic rats the activity was 33.7% and 62.1% dosed at 200 mg/kg and 400 mg/kg respectively. The above effects were observed at the 12th hour post-administration. The heavy presence of alkaloids, tannins and some lipids in this extract suggests its activity which is consistent with the report presented by Yogesha Mohan et al. [12] The effects at 400 mg/kg dose had significant antidiabetic effect (P<0.05). Several in vitro studies and one in vivo experiment observed that A. muricata inhibited the activities of α-amylase and α-glucosidase; the enzymes responsible for the breakdown of starch in the body. This indicates the suppression of postprandial hyperglycemia, [14-16]which is consistent with a low BGL usually observed after consumption of A. muricata byman.
Table 3.1: Effect of A. muricata organic extracts on blood glucose levels (mg/kg) in normoglycemic rats.
Treatment Groups |
Before Treatment |
After Treatment |
Maximal Reduction |
% Maximal Reduction |
||||||
0h |
1h |
2h |
4h |
6h |
8h |
12h |
||||
Untreated |
77.3 ± 5 |
77.7 ± 3 |
79.7 ± 5 |
83.3 ± 5 |
91.6 ± 6 |
91.7 ± 4 |
76.7 ± 7 |
0.7 |
0.9 |
|
A. muricata |
||||||||||
200mg/kg |
86.0 ± 6 |
77.3 ± 1 |
78.7 ± 8 |
76.0 ± 3 |
68.3 ± 5 |
72.0 ± 1 |
55.7 ± 6 |
30.3 |
35.3 |
|
400mg/kg |
70.3 ± 5 |
83.7 ± 7 |
78.7 ± 9 |
75.7 ± 1 |
64.7 ± 5 |
48.3 ± 3 |
24.0 ± 3* |
46.3 |
65.9 |
|
DMSO (0.3ml |
65.7 ± 1 |
70.0 ± 2 |
70.0 ± 1 |
66.7 ± 2 |
68.3 ± 2 |
69.0 ± 1 |
65.0 ± 2 |
0.7 |
1.0 |
|
Glibenclamide (10mg/kg) |
79.0 ± 4 |
73.0 ± 4 |
63.3 ± 1 |
43.3 ± 4 |
31.3 ± 3* |
13.3 ± 1* |
Lo |
65.7 |
83.3 |
|
* = p<0.05 ≥ 200mg/dL FBGL = Diabetic
Table 3.2: Effect of A. muricata organic extracts on blood glucose levels (mg/kg) in normoglycemic rats.
Treatment Groups |
Before Treatment |
After Treatment |
Maximal Reduction |
% Maximal Reduction |
||||||
1h |
2h |
4h |
6h |
8h |
12h |
|||||
Untreated |
77.3 ± 5 |
77.7 ± 3 |
79.67 ± 5 |
83.3 ± 5 |
91.6 ± 6 |
91.7 ± 4 |
76.7 ± 7 |
0.7 |
0.9 |
|
Alloxan (150mg/kg) |
69.0 ± 6 |
475.5 ± 2 |
47.3 ±2 |
514.8 ± 1 |
509.3 ± 8 |
495.3 ± 4 |
477.0 ± 2 |
467.1 ± 8 |
8.4 |
1.8 |
A. muricata |
||||||||||
200mg/kg |
62.5 ± 3 |
401.8 ± 5 |
404.8 ± 2 |
382.3 ± 1 |
340.0 ± 20 |
312.0 ± 4 |
323.0 ± 9 |
266.3 ± 2 |
135.5 |
33.7 |
400mg/kg |
82.3 ± 2 |
502.8 ± 4 |
445.0 ± 5 |
397.0 ± 4 |
321.0 ± 3 |
271.0 ± 3 |
250.5 ± 4* |
190.8 ± 5* |
312.0 |
62.1 |
DMSO (0.3ml) |
76.3 ± 4 |
398.5 ± 6 |
400.5 ± 8 |
397.3 ± 6 |
383.8 ± 1 |
398.3 ± 9 |
398.3 ± 7 |
401.0 ± 6 |
14.8 |
3.7 |
Glibenclamide (10mg/kg) |
85.5 ± 2 |
446.0 ± 2 |
311.8 ± 3 |
231.8 ± 7 |
204.3 ± 7* |
135.3 ± 1* |
104.0 ± 3* |
86.0 ± 7* |
360.0 |
80.7 |
* = p<0.05 ≥ 200 mg/dL FBGL = Diabetic.
Effect of R. parviflora root bark aquoeus extracts on blood glucose levels (mg/kg) in normoglycemic and alloxan-treated diabetic rats.
Tables 4.1 and 4.2 demonstrates the effect of aqueous extracts of R. parviflora on the FBGL +- SEM in normoglycemic and hyperglycaemic rats hyperglycemic rats respectively. Antidiabetic effects of 7.8% and 21.8% were shown in normoglycemic rats whereas in hyperglycemic rats, 10.6% and 22.1% activity were shown, at 200 mg/kg and 400 mg/kg respectively in each treatment group. The observed effects at different doses yielded a non-consistent dose-activity relationship and minimal anti-diabetic activity as well.
Table 4.1: Effect of R. parviflora aquoeus extract on blood glucose levels (mg/kg) in normoglycemic rats.
Treatment Groups |
Before Treatment |
After Treatment |
Maximal Reduction |
% Maximal Reduction |
||||||
0h |
1h |
2h |
4h |
6h |
8h |
12h |
||||
Untreated |
77.3 ± 5 |
77.7 ± 3 |
79.7 ± 5 |
83.3 ± 5 |
91.6 ± 6 |
91.7±4 |
76.7 ± 7 |
0.7 |
0.9 |
|
R. parviflora |
||||||||||
200mg/kg |
85.3 ± 3 |
130.7 ± 3 |
119.7 ± 2 |
78.7 ± 4 |
86.0 ± 5 |
88.3±5 |
107.0 ± 4 |
6.6 |
7.8 |
|
400mg/kg |
64.3 ± 2 |
79.3 ± 12 |
87.0 ± 6 |
50.3 ± 4 |
52.0 ± 3 |
59.3±3 |
59.0 ± 5 |
14.0 |
21.8 |
|
DMSO (0.3ml) |
65.7 ± 1 |
70.0 ± 2 |
70.0 ± 1 |
66.7 ± 2 |
68.3 ± 2 |
69.0±1 |
65.0 ± 2 |
0.7 |
1.0 |
|
Glibenclamide (10mg/kg) |
79.0 ± 4 |
73.0 ± 4 |
63.3 ± 1 |
43.3 ± 4 |
31.3 ± 3* |
13.3±1* |
Lo |
65.7 |
83.3 |
|
* = p<0.05 ≥ 200mg/dL FBGL = Diabetic.
Table 4.2: Effect of R. parviflora aqueous extract on blood glucose levels (mg/kg) in diabetic rats
Treatment Groups |
Before Treatment |
After Treatment |
Maximal Reduction |
% Maximal Reduction |
||||||
0h |
1h |
2h |
4h |
6h |
8h |
12h |
||||
Untreated |
77.3 ± 5 |
77.7 ± 3 |
79.7± 5 |
83.3±5 |
91.6 ± 6 |
91.7 ± 4 |
76.7 ± 7 |
0.7 |
0.9 |
|
R. parviflora |
||||||||||
Alloxan 150mg/kg |
69.0 ± 6 |
475.5 ± 2 |
47.3 ± 2 |
514.8 ± 1 |
509.3 ± 8 |
495.3 ± 4 |
477.0 ± 2 |
467.1 ± 8 |
8.4 |
1.8 |
200mg/kg |
60.8 ± 4 |
371.8 ± 12 |
354.8 ± 3 |
340.0 ± 7 |
332.3 ± 6 |
354.5 ± 14 |
346.3 ± 10 |
401.0 ± 11 |
39.5 |
10.6 |
400mg/kg |
64.5 ± 5 |
425.3 ± 11 |
359.3 ± 16 |
338.3 ± 9 |
331.3 ± 7 |
337.0 ± 7 |
379.8 ± 12 |
394.5 ± 12 |
94.0 |
22.1 |
DMSO (0.3ml) |
76.3 ± 4 |
398.5 ± 6 |
400.5 ± 8 |
397.3 ± 6 |
383.8 ± 1 |
398.3 ± 9 |
398.3 ± 7 |
401.0 ± 6 |
14.8 |
3.7 |
Glibenclamide (10mg/kg) |
85.5 ± 2 |
446.0 ± 2 |
311.8 ± 3 |
231.8 ± 7 |
204.3 ± 7* |
135.3 ± 1* |
104.0 ± 3* |
86.0 ± 7* |
360.0 |
80.7 |
* = p<0.05 ≥ 200mg/dL FBGL = Diabetic
Effect of Rutidea parviflora root bark organic extracts on blood glucose levels (mg/kg) in normoglycemic and alloxantreated diabetic rats.
The results in Tables 5.1 and 5.2 reflect the anti-diabetic activity of the organic extract of R. parviflora on the FBGL+-SEM in the normoglycemic and hyperglycemic groups respectively. In the normoglycemic groups, the blood glucose levels were reduced by 26.8% and 52.4%, 4 hours post administration of the extract at doses of 200 mg/kg and 400 mg/kg respectively. Effects of 24.6% and 46.1% were recorded in the diabetic rats, 4 hours post-administration of the organic extracts at 200mg/kg and 400 mg/kg respectively.
Table 5.1: Effect of R. parviflora root bark organic extract on blood glucose levels (mg/kg) in normoglycemic rats
Treatment Groups |
Before Treatment |
After Treatment |
Maximal Reduction |
% Maximal Reduction |
||||||
0h |
1h |
2h |
4h |
6h |
8h |
12h |
||||
Untreated |
77.3 ± 5 |
77.7 ± 3 |
79.7 ± 5 |
83.3 ± 5 |
91.6 ± 6 |
91.7 ± 4 |
76.7 ± 7 |
0.7 |
0.9 |
|
R. parviflora |
|
|||||||||
200mg/kg |
76.0 ± 4 |
73.3 ± 7 |
70.3 ± 5 |
55.7 ± 7 |
59.3 ± 5 |
66.0 ± 5 |
68.7 ± 3 |
20.3 |
26.8 |
|
400mg/kg |
90.3 ± 1 |
82.3 ± 2 |
72.7 ± 5 |
43.0 ± 2* |
60.3 ± 1 |
65.0 ± 4 |
74.1 ± 2 |
47.3 |
52.4 |
|
DMSO (0.3ml) |
65.7 ± 1 |
70.0 ± 2 |
70. 0± 1 |
66.7 ± 2 |
68.3 ± 2 |
69.0 ± 1 |
65.0 ± 2 |
0.7 |
1.0 |
|
Glibenclamide (10mg/kg) |
79.0 ± 4 |
73.0 ± 4 |
63.3 ± 1 |
43.3 ± 4 |
31.3 ± 3* |
13.3 ± 1* |
Lo |
65.7 |
83.3 |
|
* = p<0.05 ≥ 200mg/dL FBGL = Diabetic
Table 5.2: Effect of R. parviflora root bark organic extract on blood glucose levels (mg/kg) in diabetic rats
Treatment Groups |
Before Treatment |
After Treatment |
Maximal Reduction |
% Maximal Reduction |
||||||
0h |
1h |
2h |
4h |
6h |
8h |
12h |
||||
Untreated |
77.3 ± 5 |
77.7 ± 3 |
79.7 ± 5 |
83.3 ± 5 |
91.6 ± 6 |
91.7 ± 4 |
76.7 ± 7 |
0.7 |
0.9 |
|
Alloxan 150mg/kg |
69.0 ± 6 |
475.5 ± 2 |
47.3 ± 2 |
514.8 ±1 |
509.3 ± 8 |
495.3 ± 4 |
477.0 ± 2 |
467.1 ± 8 |
8.4 |
1.8 |
R. parviflora |
||||||||||
200mg/kg |
63.3 ± 2 |
560.0 ±12 |
587.0 ± 4 |
508.8 ± 4 |
422.0 ± 7 |
488.0 ± 6 |
499.0 ± 14 |
516.0 ± 9 |
138.0 |
24.6 |
400mg/kg |
68.5 ± 4 |
531.5 ± 13 |
471.0 ± 9 |
390.5 ± 6 |
286.8 ± 5* |
302.5 ± 3 |
311.3 ± 9 |
363.0 ± 16 |
244.8 |
46.1 |
DMSO (0.3ml) |
76.3 ± 4 |
398.5 ± 6 |
400.5 ± 8 |
397.3 ± 6 |
383.8 ± 1 |
398.3 ± 9 |
398.3 ± 7 |
401.0 ± 6 |
14.8 |
3.7 |
Glibenclamide (10mg/kg) |
85.5 ± 2 |
446.0 ± 2 |
311.8 ± 3 |
231.8 ± 7 |
204.3 ± 7* |
135.3 ± 1* |
104.0 ± 3* |
86.0 ± 7* |
360.0 |
80.7 |
* = p<0.05 ≥ 200mg/dL FBGL = Diabetic
It was observed that the performance of the extracts in the normoglycemic and hyperglycemic groups where not significantly different and the maximum effects were recorded at the 4th hour. There was a slight activity of R. parviflora aqueous extracts; however a moderate activity was recorded in the organic extract. It may be suggested that R. parviflora could boost the activity of A. muricata by synergism when co-administered and thus provide better therapeutic outcomes.
Comparing the anti-diabetic activity of A. Muricata and R. Parviflora
A close look at figures 1 and 2 revealed that the maximum activities of the individual plants were found to be effected by their organic extracts: It was observed that the organic extract contained the bulk of most of the secondary metabolites hence; activity can be related to this trend. This observation is in the affirmative with the works done by other researchers who reported tannins, alkaloids, flavonoids and saponins to have BGL reduction activities [17,18].
Their anti-hyperglycemic activities were more pronounced in the normoglycemic groups than in the hyperglycemic groups. Extracts of R. parviflora showed its maximum effect at the 4th hour compared to the extracts of A. muricata having their maximum effect at the 12th hour: this indicates that the plant is short acting, relatively. This phenomenon may partly be explained by the relatively low concentrations of tannins, flavonoids and saponins in the extracts. Also, other studies have reported a number of encouraging metabolic parameters on the leaf extracts of A. muricata in vivo. The documented findings include significant decrease in BGL as observed from this study, better serum lipid profile and increased body weight [19]. The possibility of preventing the destruction of pancreatic β-cells and their regeneration by A. muricata has been reported [14]. This potential benefit of A. muricata could boost insulin production in the body.
The results of toxicity studies conducted by two independent research teams demonstrated that A. muricata had good safety profile. LD50 of 1918.33 mg/kg and >5000 mg/kg were reported for the leaf extracts. Sub-chronic toxicity indicated diarrhea and increased heart rate as the only adverse effects observed 10, 20. Sherif and his colleagues suggested a potential hepato-protective and neuphro-protective activity displayed by the methanol leaf extract investigated [20].
Conclusion
According to WHO reports, in the last four decades, the burden of DM has almost quadrupled resulting from obesity, overweight and sedentary lifestyles, which has led to more adults in the age bracket of 20-79 years living with DM. Furthermore, in 2012, an estimated 2.2 million deaths associated with the increased risk of cardiovascular diseases were linked to high BGL. The astronomical cost of managing diabetes can be catastrophic to developing nations, where affordable healthcare is beyond the reach of many. This underscores the relevance of researches into medicinal plants which are cheaper, readily available and efficacious in the management of DM [1].
A. muricata, is one plant that has demonstrated significant anti-diabetic activity, in vitro, in vivo and in silico studies [21] as documented in literature. This current study reported significant p>0.05 from the 400 mg/kg leaf extract. The plant has also shown promise as a hepato-protective and neuphro-protective agent with good safety profile. However, further studies are required to identify the compounds responsible for the demonstrated activity and clinical data to support its use.
References
- WHO. Diabetes. 2020.
- IDF Diabetes Atlas, 8th edition 2017. International Diabetes Federation. 2017.
- Zhang P, Gregg E. Global economic burden of diabetes and its implications. Lancet Diabetes Endocrinol. 2017; 5: 404-405.
- Moghadamtousi SZ, Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int J Mol Sci. 2015; 16: 15625–15658.
- Rady I, Bloch MB, Chamcheu RN, Banang Mbeumi S, Anwar MR, et al: Anticancer Properties of Graviola (Annona muricata): A Comprehensive Mechanistic Review. Oxidative medicine and cellular longevity. 2018; 1826170.
- Ramasamy KPG, Doraisamy U, Mannu J, Rajamani K, Murugesan JR. Polyketide Natural Products, Acetogenins from Graviola (Annona muricata L.), its Biochemical, Cytotoxic Activity and Various Analyses Through Computational and Bio-Programming Methods. Curr Pharm Des. 2016; 22: 5204-5210.
- Angiosperm Phylogeny Website, Retrieved, (Rutidea parviflora). 2014.
- Thomas, G. High Throughput Extraction of Plant, Marine and Fungal Specimens for Preservation of Biologically Active Molecules. Molecules. 2010; 15: 4526.
- Trease and Evans Pharmacognosy. 15th Ed, India: Elseiver. 1989; 191-393.
- Agu KC, Okolie NP, Eze I, Anionye JC, Falodun A. Phytochemical analysis, toxicity profile, and hemomodulatory properties of Annona muricata (Soursop). Egypt J Haematol. 2017; 42: 36.
- Gavamukulya Y, Abou-Elella F, Wamunyokoli F, AEl-Shemy H. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac J Trop Med. 2014; S355-S363.
- Yogesha M, Jesuthankaraj NG, Thangavelu NR. Anti-diabetic and Anti-oxidant Properties of Triticum aestivum in StreptozotocinInduced Diabetic Rats, Advances in Pharmacological and Pharmaceutical Sciences. 2013: 716073.
- Kossouoh C, Mouduchirou M, Adjakidje V. Essential oil chemical composition of Annona muricata L. leaves from Benin. J Essent Oil Res. 2007; 19: 307-309.
- Adewole S, Ojewole J. Protective effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr J Tradit Complementary Altern Med. 2009; 6: 30-41.
- Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010; 11: 1365-1402.
- Agu KC, Eluehike, Ofeimun RO, Abile D, Ideho G, et al. Possible anti-diabetic potentials of Annona muricata (soursop): Inhibition of α-amylase and α-glucosidase activities. Clinical Phytoscience. 2019; 5: 21.
- Ivorra MD, Paya M, Villar A. A review of natural products and plants as potential anti-diabetic agents. J Ethnopharmacol. 1989; 27: 243-275.
- Karawya MS, Wahab SA. Diphenylamine, an antihyperglycaemic agent from onion and tea. J Natl Prod. 1984; 47: 775–780.
- Adeyemi DO, Komolafe OA, Adewole OS, Obuotor EM, Adenowo TK. Antihyperglycemic activities of Annona muricata (Linn). Afr J Tradit Complementary Altern Med. 2009; 6: 62-69.
- Sherif HB, Baba G, Abdullahi SM. Acute and Sub-Chronic Toxicity Profile of Annona Muricata (Soursop) on Wister Albino Rats. Bayero Journal of Pure and Applied Sciences. 2017; 10: 57–63.
- Alwan IB, Lim V, Samad NA, Widyawat T, Yusoff NA. Effect of Annona Muricata L. on Metabolic Parameters in Diabetes Mellitus: A Systematic Review. Current Research in Nutrition and Food Science. 2020; 08: 01-11.