Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
COVID-19: From viral infection to pulmonary failure
Guilherme Antonio de Souza Silva1; Suéllen Pedrosa da Silva2; Abdênego Rodrigues da Silva2; Georon Ferreira de Sousa1; Bárbara Rafaela da Silva Barros1; Rodrigo Cesar Abreu de Aquino1; Igor Wesland Assunção de Sá3; Fábio Augusto da Cunha Rodrigues3Evônio de Barros Campelo Júnior3; Antonio Carlos de Freitas4; Cristiane Moutinho Lagos de Melo1*
1Laboratory of Immunological and Antitumor Analysis, Department of Antibiotics, Bioscience Center, Federal University of Pernambuco, Brazil.
2 Laboratory of Protein Biochemistry, Department of Biochemistry, Bioscience Center, Federal University of Pernambuco, Brazil.
3 Clinical Hospital, Department of Tropical Medicine, Federal University of Pernambuco, Brazil.
4 Laboratory of Molecular Studies and Experimental Therapy, Department of Genetics, Bioscience Center, Federal University of Pernambuco, Brazil.
*Corresponding Author : Cristiane Moutinho
Lagos de Melo
Department of Antibiotics, Federal University of
Pernambuco, 50740-525, Recife, Brazil.
Email: crismout_melo@hotmail.com
Received : Mar 15, 2021
Accepted : Apr 20, 2021
Published : Apr 23, 2021
Archived : www.jcimcr.org
Copyright : © Melo CMLD (2021).
Abstract
SARS-CoV-2 is a virus which promoted a worldwide pandemic outbreak in 2020. The virus is highly infectious and is able to contaminate a lot of people in a short time period. The disease promoted by the virus, named COVID-19, can cause different symptoms such as fever, cough, muscle pain, headache, prostration, diarrhea, neurological complications, dermic manifestations, pulmonary impairment, dyspnea, coagulopathies, organ failure, and death. Here, we show how the infection occurs and the major characteristics observed in the lungs of patients with COVID-19. In addition, we explored the immunological activation in this environment by the virus and some treatments used in the severe phase of the disease.
Keywords: COVID-19; Pulmonary impairment; SARS-CoV-2; Virus; Pandemic.
Citation: Melo CMLD, Silva GADS, Silva SPD, Silva ARD, Sousa GFD, et al. COVID-19: From viral infection to pulmonary failure. J Clin Images Med Case Rep. 2021; 2(2): 1064.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARSCoV-2), cause of the outbreak of severe pneumonia, called COVID-19 (Coronavirus Disease 2019) [1], is present in more than 200 countries [2-4]. According to a notification from the COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University, by March 27, 2021, there were a total of 126,280,989 cases and 2,769,934 deaths worldwide [5].
The SARS-CoV-2 is believed to be able to disseminate through contaminated objects, respiratory droplets, and aerosols, where the virus can be suspended in the air for up to three hours [6,7]. The symptoms are commonly recognized as fever, dry cough, tachypnea, shortness of breath, sore throat, sneezing, nasal congestion, and other symptoms, including severe inflammatory responses with the evolution of cytokine storm, pneumonia, and sepsis [8,9].
Acute Respiratory Distress Syndrome (ARDS) is the most common complication in patients with COVID-19, with Acute Lung Injury (ALI) being the most serious form of viral infection [10]. Due to the clinical importance associated with the establishment and worsening of lung injuries in patients diagnosed with COVID-19, many studies have invested daily in testing prophylactic and therapeutic methods of treatment against acute lung injuries, through the administration of empirical antibiotics, antiviral drugs and systemic corticosteroids [6].
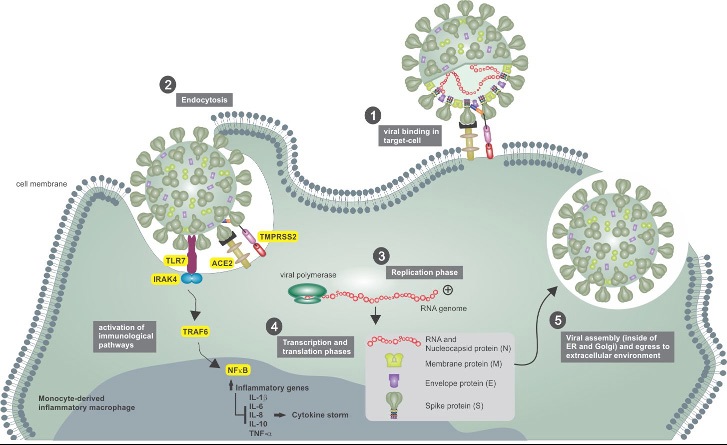
Cellular infection promoted by SARS-CoV-2 in respiratory tract cells
The upper airways are important entry regions of airborne pathogens, such as viruses, bacteria, and fungi. Some of these pathogens use this first environment to begin their multiplication process. The SARS-CoV-2 virus infects the host through ciliated and goblet cells in the nasopharyngeal region, using those cells for its replication and subsequent proliferation to other body regions [11]. Furthermore, the angiotensin-converting enzyme 2 (ACE2) is one of the receptors, present in those cells, that allow for viral entrance and favor viral fusion with the cellular membranes [12,13].
After virus-target cell fusion, the transmembrane serine protease type II (TMPRSS2) cleaves ACE2 and generates activation and conformational changes in virus protein S (through cleavage of the S1/S2 and S2’ sites), allowing the virus to fully enter cells. Inside the cell, the virus will subsequently release its genetic material ((+)ssRNA) into the cytoplasm and will use the cellular compounds to translate the viral proteins. In this context, both proteins (TMPRSS2 and ACE2) are the main determinants of the virus infection in the host [14-16].
Sungnak et al. (2020) [17,18] have shown that nasal epithelial cells present in the nasopharyngeal region, specifically goblet and ciliated cells, exhibit high expression of ACE2, followed by alveolar cells producing type 2 surfactant in the lower respiratory tract. This implies that the respiratory tract, especially the upper region, is an important repository for colonization of the virus at the beginning of infection [19,20].
ACE2 is also present in different organs and tissues of the body such as intestinal epithelial cells, in cardiac cells, in the vascular endothelium and in syncytiotrophoblastic cells in the placenta [21-23]. Moreover, at lower and non-ubiquitous levels, ACE2 is expressed in immune cells, such as monocytes/macrophages and T cells. To SARS-CoV-2 infection it is important to emphasize that other receptors and/or phagocytosis of viruses containing immune complexes may also be involved (Figure 1) [4,24,25].
Epithelial cells and macrophages when in contact with viral RNA normally activate Toll-like receptors (TLR) (3 and/or 7) in endosomes and cytosolic RNA sensors (RIG-I and MDA-5). This activation causes antiviral cellular events mediated by factors associated with the TNF receptor (TRAF). However, in SARSCoV-2 infection, there is an effective suppression of the activation of factors associated with TRAF3 and 6, limiting the activation of the NFκ-B and IRF3/7 transcription factors, suppressing early pro-inflammatory responses by type-I Interferons (IFN) and stimulating other pro-inflammatory effector cytokines such as IL-1, IL-6 and TNF-α. In addition, SARS-CoV-2 also inhibits the activation of STAT transcription factors in response to activation of the type-I interferon receptor (IFNR), which further limits the mechanisms of antiviral response (Figure 1). Those parameters may be involved in the delay of virus containment in the host organism due to lower recruitment of immune cells and activation of immune checkpoints [26,27].
SARS-CoV-2 and its interaction with immune cells in the respiratory tract
Tissue monocyte/macrophages express less ACE2, this makes them less likely to be infected by this pathway. However, immune complexes consisting of circulating antibodies, recognize the virus particles and can be absorbed by macrophages through Fc gamma receptors (FcγR), resulting in their infection. This process is known as an Antibody-Directed Enhancement (ADE) and occurs due to interaction between virions complexed with antibodies, complement components, and target immune cells [28,29]. COVID-19 has been demonstrating a significant ADE response. These disease dynamics happen due to immunological antiviral program suppression, when virions inhibit type-I IFN signaling in infected macrophages, allowing pro-inflammatory expression of IL-1, IL-6 and TNF-α cytokines, which contributes to hyperinflammation and cytokine storm syndrome [30,31].
Huang et al., (2020) [20] found in an analysis of plasma from 41 patients with COVID-19 suffering from a cytokine storm, high levels of cytokines IL-1β, IL-6, IL-7, IL-8, IL-9, IL-10, FGF, G-CSF, GM-CSF, IFN -γ, IP-10, MCP-1, MIP-1A, MIP1-B, PDGF, TNF-α and VEGF in patients admitted to an Intensive Care Unit (ICU). All patients included in the study had pneumonia and 1/3 of the patients were admitted to the ICU, where six of them died. IL-6 may be the major cytokine involved in the cytokine storm in COVID-19. A retrospective multicenter study of 150 patients with COVID-19 in China evaluated predictors of mortality for COVID-19 and showed that 68 deaths present higher levels of IL-6 [32]. Gao et al., (2020) [33] who evaluated 43 patients in China, also reported high levels of IL-6 in 33 patients.
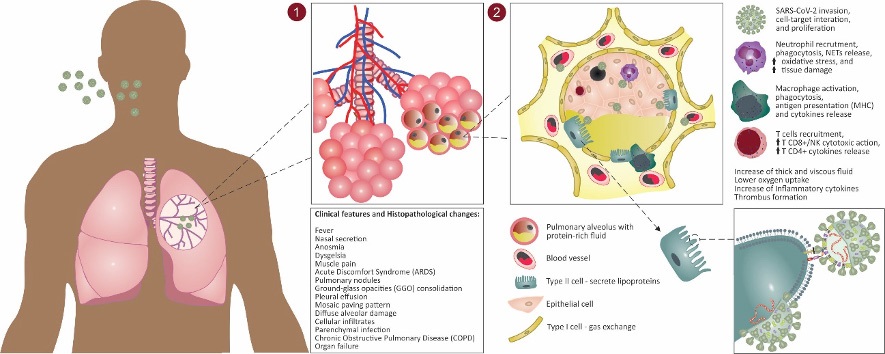
The IL-1, TNF-α and IL-6 cytokines are produced by several cells, such as tissue macrophages, mast cells, endothelial and epithelial cells. The cytokine storm promotes in an influx of macrophages, neutrophils, and T cells from the circulation to the infection site with destructive effects on host tissues [34- 37]. These higher inflammatory signals may result in destabilizing endothelial cell interactions, damaging the vascular barrier and diffuse alveolar capillarity, lowering oxygen saturation levels, resulting in failure of several organs and death [38,39].
Cytokines also regulate the activity of neutrophils and induce the expression of chemoattractants to those cells. In this context, as a result of the cytokine storm in the airways of patients with COVID-19, extracellular DNA was observed, partly originating from NETs (Neutrophil Extracellular Traps) released in response to persistent lung infections [2]. In addition, the excessive formation of NETs makes the mucus thick and viscous, not only impairing ventilation, but also facilitating the colonization of bacteria and, consequently, decreasing the patient’s respiratory function [40-43].
The evident neutrophilia also brings as a consequence the formation of microvascular thrombi by the release of NETs by neutrophils mixed with platelets, evidenced in the post-mortem examination of the lungs of patients with COVID-19 (especially the patients who present rapid disease progression and sudden death), and in tracheal aspirates of intubated ICU patients [44-48].
Although lymphocytes are blood circulation cells, they could be attracted to the inflammatory sites and display an increased immunological response. In fact, T cells play an important role in antiviral immunity, such as CD8+ T and NK cells that are capable of secreting a series of molecules such as perforin, granzymes and IFN-γ to eliminate the virus and the infected cells, and CD4+ T cells that can regulate T, B, and phagocytic cells, increasing their ability to eliminate different pathogens [49,50]. COVID-19 progression has shown a widespread lymphopenia status. Different studies point to possible T-cell exhaustion, due to the persistence of viral infection in the host, resulting in loss of cytokine production, lymphocyte apoptosis, and reduction of immune function [51-53]. Moreover, COVID-19-associated lymphopenia is promoted by NK cell reduction due to the increase of NKG2 expression (type C lectin receptor), which can stimulate or inhibit the cytotoxic activity of NK cells, being an important marker of exhaustion of these cells [54-56].
Histopathological findings of patients with COVID-19
The pathological and histological changes associated with the respiratory tract of patients infected by SARS-CoV-2 are poorly understood, so we have gathered some clinical information to facilitate the process of understanding. Ground-Glass Opacities (GGO) consolidation, pleural effusion and crazy-paving pattern are anomalies commonly found in pulmonary histopathology of COVID-19 patients [4,57,58].
Guan et al. (2020) [59], for example, demonstrated in a study of 47 patients with COVID-19 through CT scans (computed tomography) that 100% of them showed GGO, 89.36% crazy-paving and 63.83% had pulmonary consolidation. Pan et al., (2020) [60]analyzing pulmonary changes during the COVID-19 disease, from the initial diagnosis to recovery, used 82 chest CT scans from 21 patients (both sexes; aged 25 to 63 years). In the first 4 days, images showed severe but non-specific pulmonary anomalies and GGO. Over subsequent days (5-8 days) an increase in the mosaic paved pattern occurred. In the last stage (9-13 days), there was a consolidation predominantly in subpleural locations (mainly affecting the peripheral third of the lung) in the lower lobes. Similar results were found by Shi et al., (2020) [61] in a study involving 81 patients with COVID-19. The authors affirmed that pulmonary anomalies could be observed in asymptomatic patients and in more serious patients the evolution from focal unilateral to diffuse bilateral GGO occurred quickly, and it progressed to, or co-existed with, consolidations during the first 3 weeks.
Pulmonary consolidation is a pathophysiological mechanism that may be present in patients with respiratory diseases, consisting of the replacement of body fluids, air by liquid or cells, or the coexistence of both in the alveoli [62]. In addition, pulmonary consolidation may be associated with the presence of diffuse alveolar damage and cellular infiltrates, and the mosaic paving pattern found in the results of chest CT may be related to hyperplasia of the inter- and intratubular interstices. These histopathological changes in pulmonary tissue, commonly found in cases of COVID-19, can also be observed in pediatric patients, even in milder cases of the disease (Figure 2).
Studies have often shown that children affected by the disease may have pulmonary anomalies, such as GGO and nodules, mostly located in the lower lobe of both lungs near the pleural area [63-65], in a study [66] with 20 pediatric patients, showed that 80% of those patients presented high levels of procalcitonin, a pre-hormone marker that may assist in the early detection of sepsis. This high level is not commonly observed in adult patients, demanding special attention for this group. Lung consolidation with surrounding halo was observed in 50% of those patients, suggesting parenchymal infection, a typical sign in pediatric patients also not often found in adults. Moreover, 15% presented pulmonary nodules and 60% GGO.
Regarding COVID-19 in children, another point is interesting to discuss, the multisystem inflammatory syndrome (MIS) in children. In a general manner, both adults and children with COVID-19 present GGO, GGO with consolidation and nodular opacities. However, bronchial wall thickening is more commonly found in pediatric patients [67]. Moreover, as mentioned above, some children present abnormal increase of inflammatory markers included serum lactate dehydrogenase, serum D-dimer, procalcitonin, thrombocytopenia, ferritin, creatine kinase, interleukin-6, cytokine storm and multiorgan failure. Clinical signals of MIS in children are dyspnea, vomiting, and diarrhea. White blood cell counts have significant lymphopenia (especially CD16+ CD56+ natural killer cells) and changes were not found in platelets or liver function markers [68-70].
Pathological findings in a study by Wang et al. (2020) [71], investigating 151 COVID-19-infected patients, suggest that alveolar-arterial oxygen tension (A-aDO2) in terms of arterial blood gas tests is significantly affected by the abnormal presence of liver enzymes (A-aDO2 in patients with increased amount of liver enzymes: 202.0 vs. A-aDO2 in patients with normal liver enzymes: 27.6, p = 0.022). As for lung damage, postmortem biopsy of 2 patients in that study demonstrated the presence of diffuse alveolar damage, desquamation and hyaline membrane, multinucleated syncytial pneumocytes, as well as interstitial mononuclear infiltrates and hemorrhage. Peréz et al., (2020) [72] identified similar pulmonary histological findings in 14 of the 20 samples analyzed, with the addition of hyperplasia, and scattered thrombi.
These injuries found in the studies mentioned above indicate severe acute lung damage. It is also important point out that diffuse alveolar damage can be triggered by the presence of different pathogens in the host organism, including viruses. This type of lung injury is the same as that is found in patients with Acute Discomfort Syndrome (ARDS) and has been described in several autopsy studies of COVID-19 patients and in the acute (<11 days) and late stage of SARS [22,73-75].
More information is needed regarding the development of COVID-19 in patients with chronic lung disease. However, Chronic Obstructive Pulmonary Disease (COPD) in patients appears to be associated with a significant increase in the risk of developing severe complications of SARS-CoV-2 infection [76,77]. COPD is linked to decreased host immunity, increased local and systemic inflammation, exacerbated mucus production and promotion of microbiome imbalance [78]. More specifically for COPD patients affected by COVID-19, there is an increase in the levels of angiotensin-converting enzyme 2 (ACE2) [79].
Major treatments to prevent or decrease lung injuries in patients with COVID-19
From the studies analyzed, different treatment approaches were observed in patients affected by the disease, such as the use of a combination of antivirals (oseltamivir, arbidol or lopinavir/ritonavir) [80], immunoglobulin therapy, use of corticoste roids [81] and empirical antimicrobial therapy [66]. Those drugs have shown relevant results in reducing pulmonary complications and mortality rates in patients with COVID-19.
No pharmacological agent is capable of promoting an efficient humoral response against SARS-CoV-2 and we have few options for treatments to the lung injuries observed in COVID-19 patients. Thus, prophylactic, and supportive therapies can be used to reduce the progression of lung injury [82,83].
Some potential drugs, used to treat other viruses, have shown promising anti-SARS-CoV-2 effects. Studies have shown that Arbidol, an antiviral used to treat flu in Russia and China, is able to promote ACE2 receptor neutralization and the drugs Oseltamivir, Zanamivir and Peramivir, used to treat Influenza A and B, impair the SARS-CoV-2 flux among neighboring cells [84]. In addition, lopinavir/ritonavir, both HIV-1 protease inhibitors, are able to improve clinical signs during COVID-19 treatment and decrease the pulmonary damage by 3CLpro protease inhibition [85]. Azithromycin is an antibiotic that has a liposoluble mechanism of action and acts as a lysosomotropic agent, which are chemical agents that interfere in the process of the entire endocytic system [86]. It has been used against the disease due to its antimicrobial action and control of opportunistic multidrug-resistant bacterial infections [87].
Dexamethasone has been used in COVID-19 treatment due to its immunosuppressive action, inhibiting the activity of inflammatory cells such as neutrophils and promoting downregulation in Th1 and Th17 cytokines [88-90].
The immunotherapies against COVID-19 can be represented by Tocilizumab, a monoclonal antibody inhibitor of interleukin-6 and its signal transduction pathway observed in the cytokine storm; by Mepolizumab, a humanized anti-CD147 antibody used for asthma and which is able to block the entry of SARSCoV-2 into the cell, and experimental studies with convalescent plasma from patients cured of COVID-19, to promote virus neutralization in the host organism [91-96].
Conclusion
SARS-CoV-2 can promote different reactions in the host organism. The virus has tropism to important physiological targets such as the neurological, respiratory, and gastrointestinal systems. Inside of those environments, the virus can promote an inflammatory reaction which can lead to death. The respiratory tract is the most affected system and presents differences between old and young people, requiring also different approaches in care. This study showed how virus infection occurs and the subsequent effects in the host. Unfortunately, we do not yet have the cure or specific treatment for COVID-19, but the significant advances in the knowledge of the disease have promoted better care of contaminated people and decreased deaths.
References
- Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020; 92: 401-402.
- Mao Y, Lin W, Wen J, Chen G. Clinical and pathological characteristics of 2019 novel coronavirus disease (COVID-19): A systematic reviews. MedRxiv. 2020; 20025601.
- Gorbalenya AE, Baker SC, Baric RS, et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses– A statement of the Coronavirus Study Group. Nature Microbiology. 2020; 937862.
- Li H, Liu L, Zhang D, Xu J, Dai H, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. The Lancet. 2020; 09: P1517- 1520.
- This data set underlying the map, provided on the CSSE GitHub (url: https://github.com/CSSEGISandData/COVID-19), is licensed under the Creative Commons Attribution 4.0 International by the Johns Hopkins University on behalf of its Center for Systems Science in Engineering. Copyright Johns Hopkins University 2020. Attribute the data to the “COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University” or “JHU CSSE COVID-19 Data” for short, and the url: https://github.com/CSSEGISandData/COVID-19.
- Lotfi M, Hamblin MR, Rezaei N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020; 508: 254-266.
- Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020; 382: 1564-1567.
- Hui DS, Azhar E, Madani TA, Ntoumi F, Kock R, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect Dis. 2020; 91: 264-266.
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di NR. Features, evaluation and treatment coronavirus (COVID-19). StatPearls, Treasure Island, FL. 2020.
- Gallelli L, Zhang L, Wang T, Fu F. Severe Acute Lung Injury Related to COVID-19 Infection: A Review and the Possible Role for Escin. J. Clin. Pharmacol. 2020; 60: 815-825.
- Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020; 181: 281-292.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181: 271-280.
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature communications. 2020; 11: 1620.
- Melo CMD, Silva GA, Melo AR, Freitas AC. COVID-19 pandemic outbreak: the Brazilian reality from the first case to the collapse of health services. Anais da Academia Brasileira de Ciências. 2020.
- De Souza Silva GA, Silva SP, Costa MAS, Silva AR, et al. SARS-CoV, MERS-CoV and SARS-CoV-2 infections in pregnancy and fetal development. Journal of gynecology obstetrics and human reproduction. 2020; 4: 101846.
- Simmons G, Zmora P, Gierer S, Heurich A, Pohlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antiviral research. 2020; 100: 605-614.
- Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature medicine. 2020; 26: 681-687.
- Sungnak W, Ni H, Christophe B, Marjin B. SARS-CoV-2 entry genes are most highly expressed in nasal goblet and ciliated cells within human airways. arXiv preprint arXiv:2003.06122, 2020.
- Chan JFW, Yuan S, Kok KH, To KKW, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020; 395: 514-523.
- Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020; 395: 497- 506.
- Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020; 11: 3572.
- Xu H, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International journal of oral science. 2020; 12: 8.
- Zhang H, et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Annals of internal medicine. 2020; 172: 629-632.
- Merad M, Martin JC. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nature Reviews Immunology. 2020.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian. Pac. J. Allergy Immunol. 2020; 38: 1-9.
- Ziegler CG, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas C, et al. (2020). SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020; 181: 1016-1035.
- Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. (2020). Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020; 158: 1831-1833.
- Negro F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis?. Swiss Medical Weekly. 2020; 150.
- Tetro JA. Is COVID-19 receiving ADE from other coronaviruses?. Microbes and infection. 2020; 22: 72-73.
- Blanco-Melo D, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020; 181: 1036-1045.
- Hadjadj J, et al. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. MedRxiv. 2020.
- Ruan Q, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive care medicine. 2020; 46: 846-848.
- Gao Y, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID‐19. Journal of medical virology. 2020; 92: 791-796.
- Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, et al. On the alert for cytokine storm: Immunopathology in COVID-19. Arthritis & Rheumatology. 2020.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395: 1033-1034.
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of theCytokine Storm’in COVID-19. Journal of infection. 2020; 80: 607-613.
- Shimizu M, Clinical features of cytokine storm syndrome, Cron R, Behrens E editors. Cytokine Storm Syndrome. Cham: Springer. 2019: 31–42.
- Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020a; 395: 507-513.
- Lai CC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. International journal of antimicrobial agents. 2020; 55: 105924.
- Fox SE, Akmatbekov A, Harbert JL, Li G, J.Q. Brown, R.S. Pulmonary and cardiac pathology in Covid-19: The first autopsy series from New Orleans. MedRxiv. 2020; 42: 1123–1125.
- Mozzini C, Girelli D. The role of Neutrophil Extracellular Traps in Covid-19: Only an hypothesis or a potential new field of research?. Thrombosis Research. 2020; 191: 26-27.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. Journal of Microbiology, Immunology and Infection. 2020.
- Wu C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA internal medicine. 2020; 180: 934-943.
- Brondani G, et al. Pulmonary intravascular coagulopathy in COVID-19 pneumonia. The Lancet Rheumatology. 2020; 2: e458.
- Levi M, Hunt BJ. Thrombosis and coagulopathy in COVID-19: An illustrated review. Research and Practice in Thrombosis and Haemostasis. 2020; 4: 744-751.
- Middleton EA, et al. Neutrophil Extracellular Traps (NETs) Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood. 2020; 136: 1169–1179.
- Skendros P, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. medRxiv. 2020; 130: 6151-6157.
- Van Dam LF, et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: A different phenotype of thrombotic disease?. Thrombosis Research. 2020; 193: 86-89.
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006; 211: 81–92.
- Zhang C, et al. NKG2A is a NK cell exhaustion checkpoint for HCV persistence. Nat. Commun. 2019; 10: 1507.
- C Fenwick, Joo V, Jacquier P, Noto A, Banga R, Perreau M, et al. T-cell exhaustion in HIV infection. Immunol Rev. 2019; 292: 149–163.
- Ng CT, Snell LM, Brooks DG. Networking at the level of host immunity: immune cell interactions during persistent viral infections. Cell Host Microbe. 2013; 13: 652–64.
- Diao B, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Frontiers in Immunology. 2020; 11: 827.
- Walter L, Petersen B. Diversification of both KIR and NKG 2 natural killer cell receptor genes in macaques–implications for highly complex MHC‐dependent regulation of natural killer cells. Immunology. 2017; 150: 139-145.
- Zheng C, et al. Time course of lung changes at chest CT during recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020; 295: 715-721.
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell host & microbe. 2020.
- Sharma R, et al. Clinical Characteristics and Differential Clinical Diagnosis of Novel Coronavirus Disease 2019 (COVID-19). In: Coronavirus Disease 2019 (COVID-19). Springer, Singapore. 2020; 55-70.
- Prado GLM, Barjud MB. Radiologia em COVID 19: Fisiopatologia e aspectos da imagem nas diferentes fases clínicas da doença. Revista da faesf, v. 2020; 4.
- Guan CS, et al. Imaging features of coronavirus disease 2019 (COVID-19): evaluation on thin-section CT. Academic radiology. 2020.
- Pan F, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020; 200370.
- Shi H, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. The Lancet Infectious Diseases. 2020.
- Krimsky WS. Induced atelectasis and pulmonary consolidation systems and methods. U.S. Patent n. 2019; 10: 886.
- Yonker LM, Shen K, Bernard TK. Lessons unfolding from pediatric cases of COVID‐19 disease caused by SARS‐CoV‐2 infection. Pediatric Pulmonology. 2020; 55: 1085.
- Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020.
- Cao Q, Chen YC, Chen CL, Chiu CH. SARS‐CoV‐2 infection in children: transmission dynamics and clinical characteristics. J. Formos. Med Assoc. 2020; 119: 670‐673.
- Xia W, et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatric pulmonology. 2020; 55: 1169-1174.
- Amei C, Junxiang H, Yuting L, Zaosong L, Dandan C, et al. Differences in Clinical and Imaging Presentation of Pediatric Patients with COVID-19 in Comparison with Adults. Radiology: Cardiothoracic Imaging. 2020; 2.
- Ansel H, Kevin C, Axel M, Mary E, Finn BM, Fiona B, Rija N, Matthew P. COVID-19 in 7780 pediatric patients: A systematic review. Clinical Medicine. 2020; 24: 100433.
- Riphagen S, Gomez X, Gonzales-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic Lancet. 2020; 7.
- Verdoni L, Mazza A, Gerasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020.
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus–Infected pneumonia in Wuhan, China. JAMA. 2020.
- Prieto-Pérez L, Fortes J, Soto C, Vidal-González A, Alonso-Riaño M, Lafarga M, et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Modern Pathology. 2020; 1-8.
- Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020.
- Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): A report from China. J Pathol. 2003; 200: 282–289.
- Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020; 15: 700–704.
- Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respiratory Medicine. 2020; 167: 105941.
- Liu Y, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020; 63: 364–374.
- Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with communityacquired pneumonia. Eur. Respir. J. 2020; 28: 346–351.
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decadelong structural studies of SARS. J. Virol. 2020.
- Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clinical Immunology. 2020; 108393.
- Wang Y, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. Journal of Hepatology. 2020.
- Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020; 8: 738-742.
- Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med. 2017; 195: 438-442.
- Oroojalian F, et al. Novel insights into the treatment of SARSCoV-2 infection: An overview of current clinical trials. International Journal of Biological Macromolecules. 2020.
- Vargas M, Servillo G, Einav S. Lopinavir/ritonavir for the treatment of SARS, MERS and COVID-19: a systematic review. European review for medical and pharmacological sciences. 2020; 24: 8592-8605.
- Alkotaji M. Azithromycin and Ambroxol as potential pharmacotherapy for ARS-CoV-2. International Journal of Antimicrobial Agents. 2020; 106192.
- Kumar GD, Mishra A, Dunn L, Townsend A, Oguadinma IC, Bright KR, Gerba CP. Biocides and novel antimicrobial agents for the mitigation of coronaviruses. Frontiers in Microbiology. 2020; 11.
- Andreakos E, Papadaki M, Serhan CN. Dexamethasone, pro‐resolving lipid mediators and resolution of inflammation in COVID‐19. Allergy. 2020; 10: 14595.
- Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. Jama. 2020; 324: 1292- 1295.
- Singh AK, et al. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician’s perspective. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020; 14: 971-978.
- Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020; 368: 473-474.
- Zhang W, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clinical Immunology. 2020; 108393.
- Bian H, et al. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. MedRxiv. 2020.
- Esposito S, Noviello S, Pagliano P. Update on treatment of COVID-19: ongoing studies between promising and disappointing results. Le. Infezioni. Med. 2020; 2: 198-211.
- Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. International journal of antimicrobial agents. 2020; 105954.
- Brown BL, McCullough J. Treatment for emerging viruses: convalescent plasma and COVID-19. Transfusion and Apheresis Science. 2020; 102790.