Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
Persistently elevated quantitative hCG levels in a patient with gestational choriocarcinoma: A case report of simultaneous de-novo renal clear cell carcinoma and gestational choriocarcinoma
Amanda Haney1; Lindsey Choi1; John E Musser2; Kristen P Bunch1*
1 Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Tripler Army Medical Center, Hawaii, USA
2 Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Tripler Army Medical Center, Hawaii, USA
*Corresponding Author : Kristen P Bunch
Department of Obstetrics and Gynecology, Division
of Gynecologic Oncology, 1 Jarrett White Road,
Tripler AMC, HI 96859, USA.
Email: kristen.p.bunch.mil@mail.mil
Received : Apr 01, 2021
Accepted : Apr 29, 2021
Published : May 04, 2021
Archived : www.jcimcr.org
Copyright : © Bunch KP (2021).
Abstract
Background: Choriocarcinoma (CCA) is a rare form of gestational trophoblastic neoplasia. Early recognition and treatment are essential to reducing morbidity and mortality. We report the first case of concurrent CCA and renal cell carcinoma.
Case: A 35-year-old female presented postpartum with heavy vaginal bleeding. She underwent a dilation and curettage and was diagnosed with CCA. The initial Human Chorionic Gonadotropin (hCG) level was greater than 225,000 mIU/mL. Multi-agent chemotherapy was initiated. The hCG plateaued and interval imaging revealed a persistent renal mass. A partial nephrectomy and hysterectomy were performed followed by complete remission.
Conclusion: Treatment of multiple primary malignancies is challenging. Renal cell carcinoma may produce hCG, as in this case. Resection of ectopic non-trophoblastic neoplasms should be considered for persistently elevated hCG in CCA.
Teaching points: 1. Persistent vaginal bleeding at a routine postpartum
visit 6-8 weeks after delivery should prompt further
investigation, including beta-hCG measurement.
2. Persistent elevations of human chorionic gonadotropin levels in patients with gestational trophoblastic
neoplasia actively receiving treatment should prompt
further inquiry into ectopic production by non-trophoblastic neoplasms.
3. A multidisciplinary approach to managing dual primary malignancies is essential to optimizing cancer
outcomes and reducing morbidity and mortality.
Citation: Haney A, Choi L, Musser JE, Bunch KP. Persistently elevated quantitative hCG levels in a patient with gestational choriocarcinoma: A case report of simultaneous de-novo renal clear cell carcinoma and gestational choriocarcinoma. J Clin Images Med Case Rep. 2021; 2(3): 1103.
Introduction
Gestational Trophoblastic Neoplasia (GTN) is a spectrum of tumors comprised of anaplastic trophoblastic tissue, including invasive moles, Choriocarcinoma (CCA), Placental Site Trophoblastic Tumor (PSTT), and epithelioid trophoblastic tumors. GTN is most commonly diagnosed after a molar pregnancy; however, diagnosis can follow any antecedent pregnancy. Gestational CCA is a specific subset of GTN that contains anaplastic cytosyncytiotrophoblasts and syncytiotrophoblasts without any chronic villi [1]. Gestational CCA is the most common form of GTN diagnosed following molar and non-molar pregnancies, as well as the most aggressive GTN histology [2]. Despite its aggressive histology, CCA is highly sensitive to chemotherapy and the overall cure rate for high-risk GTN is greater than 90% [3].
Of female reproductive system cancers, gestational trophoblastic tumors account for less than 1% [4]. In a symptomatic patient, the most common presentation is metastatic disease [4]. This is likely due to the fragile vessels in the metastatic trophoblastic lesions that can cause bleeding at various sites. Signs and symptoms include hemoptysis, intraperitoneal or pelvic bleeding, and acute neurological deficits from increased intracranial pressure or bleeding [1].
The most common metastatic sites are the lungs (80%), vagina (30%), brain (10%) and liver (10%) [5]. Any patient diagnosed with CCA should have a thorough assessment for proper staging and treatment. At a minimum, these patients should undergo a complete history and physical, Human Chorionic Gonadotrophin (hCG) level monitoring, hepatic/thyroid/renal function tests, and a complete blood count. To evaluate the full extent of disease, chest radiography should be obtained to assess for metastatic disease as well as a Computed Tomography (CT) scan or Magnetic Resonance Imaging (MRI) of the head and abdomen, when indicated. Typically, new masses found on imaging are thought to represent metastatic disease. Biopsies to confirm the diagnosis are not recommended given the risk of significant bleeding that may occur. In rare cases, as reported here, a secondary primary malignancy can be present.
Case presentation
A 35-year-old gravida 9 para 3-1-5-4 female had an uncomplicated vaginal delivery of live born neonate with a macroscopically normal appearing placenta. Her obstetrical history included 1 preterm and 3 term spontaneous vaginal deliveries, and five first trimester spontaneous abortions. All her pregnancies were uncomplicated except for a postpartum hemorrhage due to uterine atony several years prior. Her most recent pregnancy and postpartum course were otherwise unremarkable.
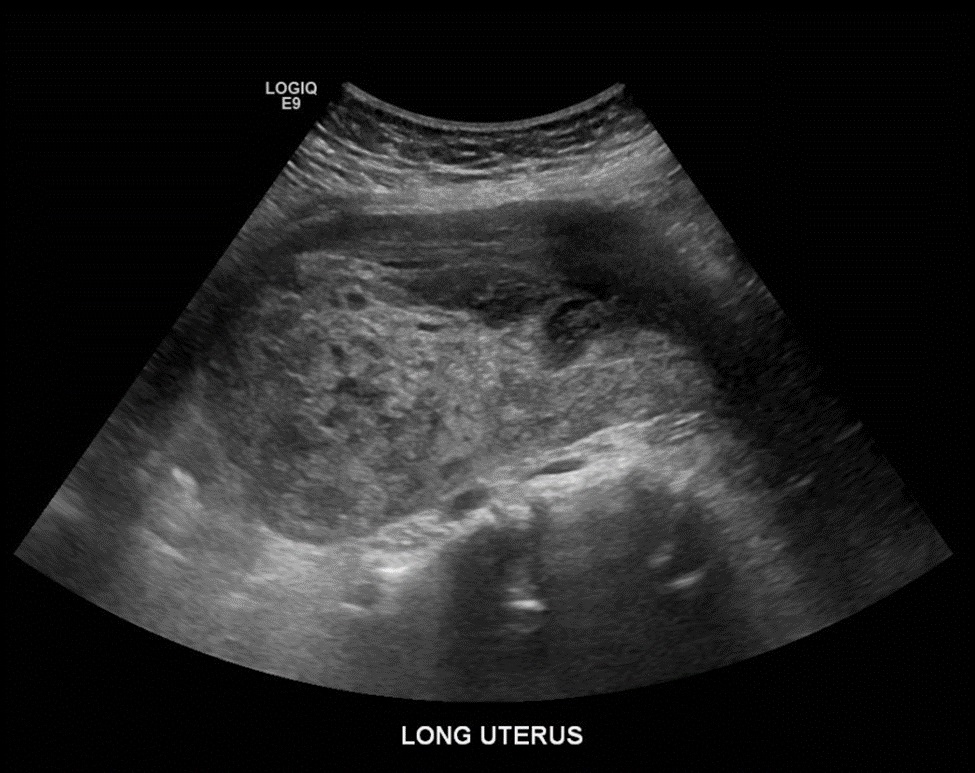
She presented to the same day evaluation clinic one month postpartum with the complaint of heavy vaginal bleeding. A bedside ultrasound confirmed a 2.9-centimeter heterogeneous mass within the uterine cavity. She was treated for suspected retained products of conception with misoprostol. Two weeks later, she presented to her routine postpartum appointment with persistent vaginal bleeding, passage of clots, abdominal pain, and new-onset nausea, vomiting, lightheadedness, and dizziness. Review of her vital signs were significant for tachycardia to 109 beats per minute with a normal blood pressure and normal temperature. On physical examination, the uterus was enlarged and she demonstrated fundal tenderness to palpation. Her hematocrit was 21.9%. A pelvic ultrasound was performed and identified a mass at the uterine fundus and heterogeneous material within the endometrial cavity, consistent with presumed retained products of conception and hemorrhage (Figure 1). The patient underwent a dilation and curettage, received a blood transfusion, and was discharged home in stable condition.
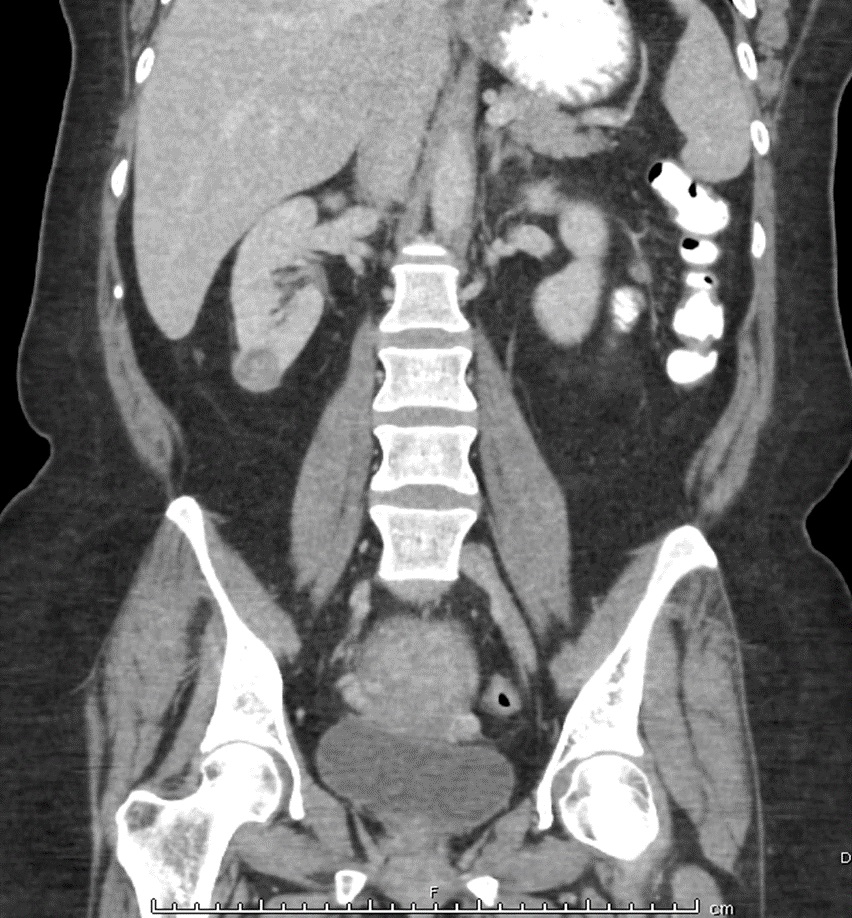
The tissue pathology was concerning for GTN with a differential diagnosis of CCA versus PSTT. Additionally, she had an elevated hCG of >225,000 mIU/mL and imaging consistent with metastatic disease. Chest radiography revealed multiple lung nodules and a Computed Tomography (CT) scan confirmed the presence of pulmonary lesions as well as the presence of a right inferior renal mass measuring 2.7 centimeters (Figure 2). MRI of the brain was negative for central nervous system lesions. She was admitted for treatment of refractory nausea with a plan to simultaneously initiate first-line multi-drug chemotherapy, EMA/CO (etoposide, methotrexate, dactinomycin, cyclophosphamide, vincristine) for presumed GTN. The endometrial tissue specimen was sent to the University of California Los Angeles for a second opinion which confirmed CCA. Presuming the renal mass represented a metastatic lesion, she had a high-risk prognostic score of at least 8 (Table 1) and stage III metastatic disease.
With the initial chemotherapy regimen, the patient’s hCG values declined appropriately and the lung nodules decreased in size, as seen on repeat chest imaging. However, the renal mass was unchanged in size, raising concern for a second primary malignancy. A renal mass biopsy was performed and confirmed the diagnosis of grade 1 renal clear cell carcinoma. After a multidisciplinary discussion with urologic oncology, the plan was to complete EMA/CO therapy until remission of her CCA, at which time urologic oncology would treat the renal carcinoma.
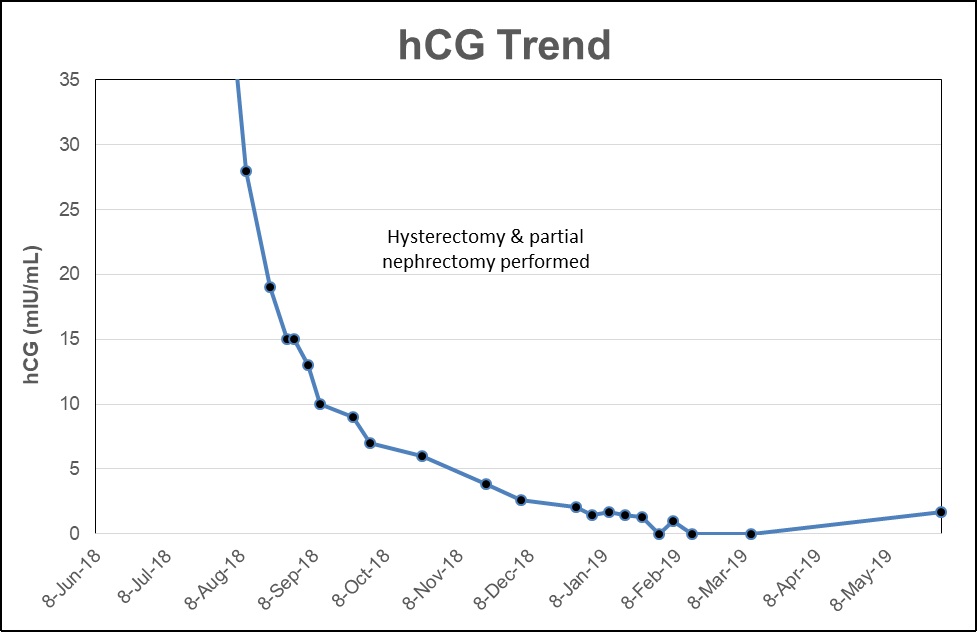
After 6 cycles of EMA/CO, the hCG level plateaued and remained persistently elevated between 7-20 mIU/ml (Graph 1). The patient was transitioned to second line chemotherapy with EMA/EP (etoposide, methotrexate, dactinomycin, etoposide, cisplatin). However, after her first cycle of EMA/EP, she reported significant tinnitus and was diagnosed with mild high frequency hearing loss. Due to the acute hearing loss, she was switched from cisplatin to carboplatin for cycles 2 and 3 of the EMA/EP regimen. Despite switching to EMA/EP, the hCG remained elevated. At this time, the renal carcinoma was considered a potential source of ectopic hCG production.
Ectopic production of hCG occurs with several non-trophoblastic tumors, including renal cell carcinoma [6-9]. A retrospective study reported that tissue expression of β-hCG occurs in 15% of renal cell carcinomas and is an independent prognostic variable [10,11]. The patient’s persistently elevated hCG was presumed to be secondary to ectopic production by the renal cell carcinoma. The patient had satisfied parity and it was recommended she undergo a minimally invasive hysterectomy and bilateral salpingectomy concurrently with a right partial nephrectomy to eliminate all potential sources of hCG production. Her hCG levels declined postoperatively and remained negative through 3 additional cycles of EMA/CO following surgery. The patient continues to be disease free from both cancers 14 months after completion of treatment.
Discussion
Multiple Primary Malignant Neoplasms (MPMN) are two or more tumors that are not an extension of, recurrence, or mestastasis of the other [12]. A review of literature of more than 1.1 million patients reported the prevalence of MPMN ranges from 0.7% to 11.7% [12]. The occurrence of multiple malignancies can pose challenges to treatment and highlights the necessity of multidisciplinary teams and shared decision making.
CCA is rare following a term delivery and is reported to occur in 1 in 150,000 normal pregnancies [1]. Berkowitz reported a 4.1% incidence of CCA in 336 cases of GTN following a term delivery [1]. Despite its rarity, CCA should be included on the differential diagnosis of a postpartum patient with corresponding complaints. The World Health Organization (WHO) published a prognostic scoring system that guides treatment options by reliably predicting the potential for chemotherapy resistance. A prognostic score of 7 or higher is considered high risk and requires a multi-drug regimen while single drug treatment is recommended for low risk GTN. Staging is completed using the International Federation of Gynecology and Obstetrics (FIGO) guidelines based on the anatomical staging and prognostic score. Stage I includes any disease confined within the uterus. Stage II comprises localized spread of disease to the vagina and/ or pelvis. Stage III encompasses metastatic disease to the lungs only. Stage IV disease involves the brain, liver, kidneys and/or gastrointestinal tract.
The first-line treatment for CCA is multi-agent chemotherapy with the EMA/CO regimen and has remission rates approaching 80-90% [1]. Treatment cycles are required every 2-3 weeks until remission, toxicities permitting. An additional 2-3 cycles are recommended after the hCG becomes negative. The second-line therapy for resistant tumors is EMA/EP during which etoposide and cisplatin are administered on cycle day 8. When given alone or in combination with surgical intervention, it has been shown to lead to remission in 76% of patients whose tumor is deemed resistant to EMA/CO [1]. Following remission, patients should be monitored through a combination in hCG levels and imaging as indicated, until negative hCG levels are sustained for 6 -24 months depending on the extent of the disease [1].
Ectopic hCG production by non-trophoblastic neoplasms has been described in multiple cases in medical literature [14]. Renal masses have been reported to commonly express hCG genes. A 2003 study by Jiang and colleagues reported 50% of both benign renal tissue and renal cell carcinomas positively express hCG genes [9].
Renal cell carcinoma accounts for 2-3% of all new adult malignancies. Due to common use of CT imaging in current medical practice, more than 50% of renal cell carcinoma is incidentally detected [15]. Clear cell histology is seen in >80% of sporadic cases of kidney tumors, and is associated with a VHL gene mutation in both sporadic and hereditary forms [16]. Our patient tested negative for a germline VHL mutation and has no family history of kidney cancer; despite her young age, this appears to be a sporadic case of renal cell carcinoma. Surgical intervention with resection or ablative methods is the treatment of choice with the best cure rates. Identifying the source of the second malignancy is important when treating MPMN, as effective therapy is often distinct for each neoplasm, as evidenced in our patient with CCA and renal clear cell carcinoma.
While infrequent, ectopic hCG production should always remain in the differential diagnosis for patients with persistent elevated hCG levels in the postpartum period. The renal mass in our patient was initially presumed to be metastatic CCA. While CCA often metastasizes to the lungs, it can also have renal system involvement. Given the multiple common sites of metastasis, it is not unusual to presume that masses found in other locations are more likely evidence of metastasis rather than MPMN. However, in the setting of persistently elevated hCG and an unchanged mass size refractory to second-line chemotherapy, further investigation is warranted.
In rare cases such as our patient, the renal mass was refractory to GTN targeted chemotherapy leading to a diagnosis of concomitant renal primary. We presume the CCA was effectively treated with EMA/CO leading to significantly lower hCG values during the initial treatment period, as evidenced by the decreased uterine mass and metastatic lung nodules. In retrospect, the persistently elevated hCG levels requiring second-line chemotherapy were from the ectopic production by the renal clear cell carcinoma.
This is the first report of ectopic hCG production by renal clear cell carcinoma in the setting of concurrent gestational CCA, and the management of both malignancies. In patients who do not have future fertility desires, removal of the renal mass and uterus simultaneously are feasible and can be performed by a minimally invasive route. This treatment approach eliminated unnecessary chemotherapy exposure and its associated toxicities, while also removing the presumed cause of the patient’s persistently elevated hCG. The multi-disciplinary approach to this patient’s care was essential in improving prognosis, reducing surgical morbidity and achieving remission.
Acknowledgements: The authors wish to thank the patient for trusting us with her care and her assistance in completing this report.
References
- Berkowitz RS, Goldstein DP. Current management of gestational trophoblastic diseases. Gynecol Oncol. 2009; 112: 654-662.
- Lee E, Cho H. A case of intraplacental choriocarcinoma with pulmonary metastasis. Case Rep Oncol 2019: 12: 802-806.
- Lurain J. Gestational trophoblastic disease II: Classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011; 204: 11-18.
- Brinton LA, Bracken MB, Connelly RR. Choriocarcinoma incidence in the United States. Am J Epidemiol. 1986; 123: 1094.
- Berkowitz RS, Goldstein DP. Pathogenesis of gestational trophoblastic neoplasms. Pathobiol Ann. 1981; 11: 391-411.
- Adekunle A, Lam A, Turbow S, Stallworth C, Ferris M, et al. Betahuman chorionic gonadotropin-producing renal cell carcinoma. Am J Med. 2016; 129: e29-31.
- Mohammed I, Turner G, Cranston D. Human chorionic gonadotropin-secreting clear cell renal cell carcinoma with paraneoplastic gynaecomastia. Scan J Urol Nephrol. 2008; 42: 555-557.
- Fukutani K, Libby J, Panko W, Scardino, P. Human chorionic gonadotropin detected in urinary concentrates from patients with malignant tumors of the testis, prostate, bladder, ureter and kidney. J Urol. 1983: 129: 74-77.
- Jiang Y, Zeng F, Xiao C, Liu J. Expression of beta-human chorionic gonadotropin genes in renal cell cancer and benign renal disease tissues. J Huazhong Univ Sci Technolog Med Sci. 2003; 23: 291-293.
- Hotakainen K, Ljungberg B, Haglund C, Nordling S, Paju A, Stenman U. Expression of the free beta-submit of human chorionic gonadotropin in renal cell carcinoma: Prognostic study on tissue and serum. Int J Cancer. 2003; 104: 631-635.
- Hotakainen K, Lintula S, Ljungberg B, Finne P, Paju A, et al. Expression of human chorionic gonadotropin beta-subunit type I genes predicts adverse outcome in renal cell carcinoma. J Mol Diagn. 2006; 8: 598-603.
- Demandante CG, Troyer DA, Miles TP. Multiple primary malignant neoplasms: Case report and comprehensive review of the literature. Am J Clin Oncol. 2003; 26: 79-83.
- Ghaemmaghami F, Zarchi MK. Early onset of gestational trophoblastic disease after full-term pregnancy. Intl J of Biomed Sci. 2008; 4: 74-77.
- Braunstein GD, Vaitukaitis JL, Carbone PP, Ross GT. Ectopic production of human chorionic gonadotrophin by neoplasms. Ann Intern Med. 1973; 78: 39-45.
- Bamias A, Escudier B, Sternberg CN, Zagouri F, Dellis A, Tzannis K, et al. Current clinical practice guidelines for the treatment of renal cell carcinoma: A systemic review and critical evaluation. Oncologist. 2017; 22: 667-679.
- Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO classification of tumours of the urinary system and male genital organs. Lyon (France): IARC Press. 2016.
- FIGO Committee on Gynecologic Oncology. Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. Int J Gynaecol Obstet 2009; 105: 3-4.