Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
MOG-related bilateral optic neuritis in a patient with cystic fibrosis
Rachelle Abi Nahed1; Georges Succarieh2; Amine Al Soueidy2; Patrick Baz2; Gabrielle Macaron1,3; Karine Abou Khaled1*
1 Neurology Department, Hotel-Dieu De France, Saint Joseph University, Faculty of Medicine, Beirut, Lebanon.
2 Ophthalmology Department, Hotel-Dieu De France, Saint Joseph University, Beirut, Lebanon.
3 Mellen Center for Multiple Sclerosis Treatment and Research, Neurological Institute, Cleveland Clinic, Cleveland, USA.
*Corresponding Author: Karine Abou Khaled
Neurology Department, Hotel-Dieu De France, Saint
Joseph University, Faculty of Medicine, Hotel Dieu de
France Hospital, Boulevard Alfred Naccache, Ashrafieh,
Beirut, Lebanon.
Email: karine.aboukhaled@usj.edu.lb
Received : May 08, 2021
Accepted : Jun 21, 2021
Published : Jun 25, 2021
Archived : www.jcimcr.org
Copyright : © Khaled KA (2021).
Abstract
Optic neuropathy secondary to Cystic Fibrosis (CF) has been described as a manifestation of chloramphenicol toxicity. Inflammatory optic neuritis and Myelin Oligodendrocyte Glycoprotein (MOG)-related disorders have not been previously described in patients with CF. We report the case of a 19-year-old woman with cystic fibrosis who presented for sub-acute onset decreased visual acuity, dyschromatopsia, and optic disk swelling in both eyes, with near-complete resolution after a course of high-dose corticosteroids. MOG-IgG were positive in the serum. MOG-related optic neuritis can occur in CF and represent a diagnostic and long-term therapeutic challenge.
Keywords: Optic neuritis; MOG; cystic fibrosis.
Citation: Khaled KA, Nahed RA, Succarieh G, Soueidy AA, Baz P, et al. MOG-related bilateral optic neuritis in a patient with cystic fibrosis. J Clin Images Med Case Rep. 2021; 2(3): 1198.
Introduction
Cystic Fibrosis (CF) is a rare autosomal recessive disease involving exocrine gland function in multiple organs particularly the lungs, leading to life threatening complications in 90% of patients [1]. The disease is secondary to a mutation in CF transmembrane conductance regulator gene, which codes for chloride and sodium channels at the cell surface epithelium [2]. Typical clinical features are chronic pulmonary changes, chronic pancreatic deficiency and gastrointestinal obstruction. Ocular pathologies are rare in CF; xerophthalmia, nyctalopia and ocular surface abnormalities occur due to vitamin A deficiency (secondary to impaired liver secretion and transport, and malabsorption) and reduced tear secretion secondary to abnormal chloride secretion [3]. Rare cases of idiopathic intracranial hypertension and chloramphenicol-induced acute bilateral optic neuropathy were reported in CF patients [4,5]. Inflammatory optic neuritis has not been reported.
We present the unique case of a patient with CF with no previous exposure to chloramphenicol presenting severe bilateral optic neuritis and positive Myelin Oligodendrocyte Glycoprotein (MOG) Immunoglobulin G (IgG) in the serum.
Case presentation
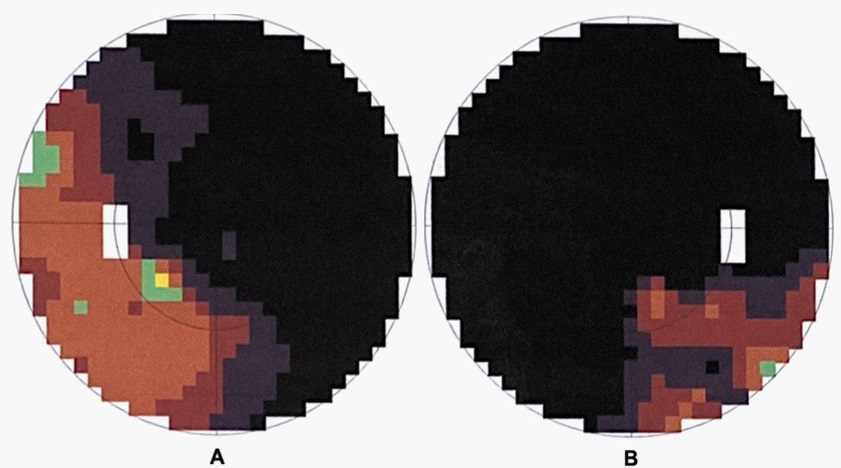
A 19-year-old women presented to the emergency department for subacute-onset decreased Visual Acuity (VA) in both eyes and retro-orbital pain, worsening over the course of 3 days. She had a past history of CF since the age of 6 months, with associated chronic pancreatic insufficiency, chronic pansinusitis, recurrent pneumonia, and iron deficiency anemia. She was treated with ipratropium/salbutamol, azithromycin, ursodiol, fluticasone propionate, tobramycin, iron and pancrelipase. Physical examination was prominent for poorly reactive pupils bilaterally, severely decreased VA in both eyes (best corrected VA 20/200 bilaterally), and bilateral dyschromatopsia (1 correct answer over 21 Ishihara plates bilaterally). Slit-lamp examination was unremarkable. Visual field testing showed severe constriction of visual fields in both eyes (Figure 1). Intra-ocular pressure measured by applanation tonometry was 13 mmHg bilaterally. Dilated fundus examination revealed bilateral blurred margins of the optic discs, without peripapillary haemorrhages, and a normal cup to disc ratio bilaterally. Visual evoked potential revealed prolonged distal latency of the P100 wave of 161 ms on the left, and absent responses on the right. Brain and orbital Magnetic Resonance Imaging (MRI) with gadolinium injection, and brain magnetic resonance angiography and venography did not show significant abnormalities, except for acute pansinusitis. Cervical spine MRI was normal. Cerebrospinal Fluid (CSF) analysis was within normal limits, including a normal CSF opening pressure. MOG-IgG in the serum using cell-based assays were positive (titre 1:20). An extensive work-up was otherwise negative (Table 1).
Table 1: Results of serum and cerebrospinal fluid analysis.
Test |
Results |
Blood work: |
|
Lupus anticoagulant |
Negative |
Beta-2 glycoprotein 1 antibody (N < 20 RU/mL) |
10 RU/mL |
Anti-cardiolipin antibody (N < 10 U/mL) |
3 U/mL |
Angiotensin converting enzyme (N: 20-70 U/L) |
38 U/L |
Aquaporin-4 IgG |
Negative |
Myelin Oligodendrocyte glycoprotein-IgG |
Positive (titre: 1:20) |
PPD Skin test |
Negative |
anti- double strand DNA antibodies |
Negative |
ANA |
Negative |
ENA Panel* |
Negative |
C3 (N: 83-193 mg/dL) |
148 |
C4 (N: 15-57 mg/dL) |
28 |
c-ANCA |
Negative |
p-ANCA |
Negative |
Vitamin B12 (N: 211-946 pg/ml) |
900 pg/ml |
Folic acid (N: 3.1-18.7 ng/ml) |
7.1 ng/ml |
EBV IgG and IgM |
Negative |
T.P.H.A and V.D.R.L |
Negative |
CMV IgG (Negative< 0.5 U/mL) |
>250 |
CMV IgM |
Negative |
HIV (1+2) serology |
Negative |
CSF Studies |
|
Opening pressure |
13 cm of H2O |
White blood cells (N: 0-5 cells/mm3) |
3 cells/mm3 |
Red blood cells (N: 0-5 cells/mm3) |
2 cells/mm3 |
Gram stain and bacterial culture |
Negative |
Protein (N: 0.15- 0.6 g/L) |
0.26 g/L |
Glucose (N: 2.5- 4.4 mmol/L ) |
3 mmol/L |
IgG index (N: 0.32-0.60) |
0.54 |
CSF oligoclonal bands |
Negative |
Cytology |
Negative for malignant cells |
ANA: Antinuclear Antibody; ANCA: Anti-Neutrophil Cytoplasmic Antibodies; C3: Complement Component 3; C4: Complement Component; CENP: Centromere Protein; CMV: Cytomegalovirus; CSF: Cerebrospinal Fluid; DNA: Deoxyribonucleic Acid; EBV: Epstein–Barr Virus; ENA: Extractable Nuclear Antigen; HIV: Human Immunodeficiency Virus; Ig: Immunoglobulin; P-ANCA: Perinuclear Anti-Neutrophil Cytoplasmic Antibodies; PPD: Purified Protein Derivative; RNP: Ribonucleoprotein Particle; RU: Relative Units; Scl-70: Anti-Topoisomerase I; Sm: Smith; SSA: Sjögren's-Syndrome-Related Antigen A; SSB: Sjögren's-Syndrome-Related Antigen B; T.P.H.A: Treponema Pallidum Hemagglutination Assay; V.D.R.L: Venereal Disease Research Laboratory. *ENA panel detects antibodies anti-Sm, anti-RNP, anti-Sm-RNP, anti-U(1)RNP, anti-SSA, anti-SSB, anti-Scl-70, anti-Jo, anti-CENP-A and anti-CENP-B.
She was treated with high-dose intravenous methylprednisolone (1 gram per day for 6 days) followed by an oral steroid taper for one week. Within a week, significant improvement was observed, with a VA of 20/60 in the right and 20/30 in the left eye, as well as improvement in colour discrimination (8/21 on the right and 12/21 on the left on Ishihara plates test). Last physical examination 6 months after onset showed normal VA, colour vision, and funduscopic exam in both eyes, and she remained clinically stable, without subsequent neurological events. Repeat MOG-IgG testing was not performed due patient refusal.
Discussion
We report the unique case of bilateral inflammatory optic neuritis in an adult patient with CF. In our case, the clinical presentation of bilateral and severe subacute visual impairment was typical of MOG-related optic neuritis. MOG-related disorders also present with longitudinally extensive transverse myelitis with predominant grey matter involvement, acute demyelinating encephalomyelopathy (ADEM)-like presentation, and brainstem/diencephalic syndromes [6]. Optic neuropathy in the setting of CF is rare and is often the consequence of prolonged therapy with chloramphenicol [5]. Other CNS manifestation of CF are rare and are related to ischemic events. In our patient, the sub-acute onset, associated retro-orbital pain, prompt response to steroids, and papilledema, all argue against an ischemic process. Autoimmune diseases reported in CF include cutaneous vasculitis with antineutrophil cytoplasmic antibodies positivity [7]. Demyelinating CNS diseases have not been observed in these patients. Only anecdotal reports of bilateral panuveitis and multiple sclerosis were found in the literature [8]. The relationship between CF and MOG-associated optic neuritis in this case is unclear. It has been postulated that MOG-autoimmunity can be triggered by a viral infection, [9] and our patient suffered from acute sinusitis prior to onset of visual symptoms. Consequently, upper and lower respiratory tract infections due to chronic immunosuppression in patients with CF may trigger anti-MOG-IgG production via molecular mimicry, but future observations and studies are needed to confirm this hypothesis. In our patient, acute sinusitis was present at diagnosis and could have been the antigenic trigger for MOG autoimmunity.
Unfortunately, repeat MOG testing was not performed in our patient, and we cannot confirm that she had persistently positive MOG-IgG. Moreover, the interpretation of low-titre positive MOG-IgG is still incompletely elucidated. In a large cohort of MOG-related ADEM cases, initial MOG-IgG titres were higher in patients with persistent MOG-IgG seropositivity compared to those with transient antibody elevation [10]. However, some patients with a typical relapsing-remitting clinical presentation have low titres at presentation, and those titres fluctuate during the disease course [10]. Due to this uncertainty, and the higher risk of infection due to CF, initiating a long-term treatment with an immunosuppressant was not pursued in our patient.
Conclusion
In conclusion, MOG-related optic neuritis can occur in patients with CF and should be in the differential diagnosis after excluding more common causes of optic nerve pathology. The decision to initiate a preventive immunosuppressant therapy in those cases is challenging and should take into consideration the presence of persistently positive MOG-IgG over time indicating a risk of relapse and the increased risk of severe infections due to chronic immunosuppression.
Disclosures: R. Abi Nahed, G. Succarieh, A. Al Soueidy, P. Baz and K. Abou Khaled: Nothing to disclose.
G. Macaron received fellowship funding from the National Multiple Sclerosis Society Institutional Clinician Training Award ICT 0002 and from Biogen Fellowship Grant 6873-P-FEL. She has served on a scientific advisory board for Genentech and has participated in educational programs for John Hopkins e-Literature review, Novartis, and Mercks.
Author Contributions
Acknowledgments: The authors would like to thank the patient for providing consent to publish her case.
Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review.
References
- Reis FJ, Damaceno N. [Cystic fibrosis]. J Pediatr (Rio J). 1998; 74: S76-94.
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995; 269: 847–850.
- Petersen RA, Petersen VS, Robb RM. Vitamin A deficiency with xerophthalmia and night blindness in cystic fibrosis. Am J Dis Child 1960. 1968; 116: 662–665.
- Lucidi V, Di Capua M, Rosati P, Papadatou B, Castro M. Benign intracranial hypertension in an older child with cystic fibrosis. Pediatr Neurol. 1993; 9: 494–495.
- Nielsen EL. Optic neuritis as a complication to treatment of cystic fibrosis with chloramphenicol. Ugeskr Laeger. 1972; 134: 2328–2399.
- Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: The MOGADOR study. Neurology. 2018 22; 90: e1858– 1869.
- Finnegan MJ, Hinchcliffe J, Russell-Jones D, Neill S, Sheffield E, et al. Vasculitis complicating cystic fibrosis. Q J Med. 1989; 72: 609–621.
- Mucoviscidose, uvéite infantile et sclérose en plaques à l’âge adulte. J Fr Ophtalmol. 2005 1; 28: 850–853.
- Macaron G, Ontaneda D. MOG-related disorders: A new cause of imaging-negative myelitis? Mult Scler Houndmills Basingstoke Engl. 2020; 26: 511–515.
- López-Chiriboga AS, Majed M, Fryer J, Dubey D, McKeon A, et al. Association of MOG-IgG Serostatus With Relapse After Acute Disseminated Encephalomyelitis and Proposed Diagnostic Criteria for MOG-IgG-Associated Disorders. JAMA Neurol. 2018; 75: 1355–1363.