Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
A novel FSHR-ALK and EML4-ALK double fusion variant in advanced lung adenocarcinoma: A case report
Yili Zhu; Ying Wu; Bo Huang; Jun Fan*; Xiu Nie*
Department of Pathology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
*Corresponding Author: Xiu Nie & Jun Fan
Union Hospital, Department of Pathology, Tongji
Medical College, Huazhong University of Science and
Technology, 1277 Jiefang Avenue, Wuhan, Hubei,
China.
Email: niexiuyishi@126.com & fanjun0915@sina.com
Received : May 28, 2021
Accepted : Jul 02, 2021
Published : Jul 07, 2021
Archived : www.jcimcr.org
Copyright : © Nie X & Fan J (2021).
Abstract
Anaplastic Lymphoma Kinase (ALK) gene fusions are detected in approximately 5% of lung adenocarcinomas, and data on double ALK fusions remain scarce because of their low occurrence rate. To date, there have been only a small number of case reports on double ALK fusions. In this report, we describe a novel double ALK fusion, FollicleStimulating Hormone Receptor (FSHR)-ALK and echinoderm microtubule-associated protein-like 4 (EML4)-ALK, which was observed in a patient with advanced lung adenocarcinoma who was sensitive to alectinib. Alectinib treatment was administered orally 300 mg twice daily. After a month, imaging re-examination revealed a significant decrease in tumour size and markedly relieved atelectasis. According to the Response Evaluation Criteria in Solid Tumours, the patient was considered to have a partial response to alectinib. To the best of our knowledge, this report is the first to describe this novel double ALK fusion in a patient, and these findings may provide a reference for other patients with such gene alterations.
Keywords: Alectinib; EML4-ALK and FSHR-ALK double fusion; nextgeneration sequencing.
Citation: Zhu Y, Wu Y, Huang B, Fan J, Nie X. A novel FSHR-ALK and EML4-ALK double fusion variant in advanced lung adenocarcinoma: A case report. J Clin Images Med Case Rep. 2021; 2(4): 1220.
Introduction
Non-Small-Cell Lung Cancer (NSCLC) is estimated to account for 80 to 85% of all lung cancers [1]. Oncogenic ALK gene rearrangements, in which the intact ALK kinase domain is fused to N-terminal fusion partners, occur in approximately 5% of patients with NSCLC [2]. ALK fusion partners have gradually been identified, including EML4, KIF5B, CMTR1, KLC1, TNIP2 and Cux1 [3,4]. Among these fusions, EML4-ALK fusion gene variants accounted for 80% of ALK fusion genes. With the development of Next-Generation Sequencing (NGS), more than 20 fusion partners for ALK in NSCLC have been identified [5]. First-generation ALK-Tyrosine Kinase Inhibitors (TKIs), such as crizotinib, are recommended as first-line treatments for ALK-rearranged NSCLC and have exhibited impressive efficacy in treating ALK-positive lung adenocarcinoma [6]. Second-generation ALK-TKIs, such as alectinib, ceritinib and brigatinib, and third-generation ALKTKIs, such as lorlatinib, have also been developed [7]. Alectinib has exhibited a longer Progression-Free Survival (PFS) in the treatment of advanced lung cancer [8]. However, the existence of an ALK double fusion in one patient with NSCLC has occasionally been reported. Moreover, ALK-TKIs are widely utilized in ALK-positive patients, but patients with different ALK fusions have different responses [9]. Here, a patient with an FSHR-ALK and EML4-ALK double fusion is described who responded to alectinib and showed changes before and after treatment.
Case report
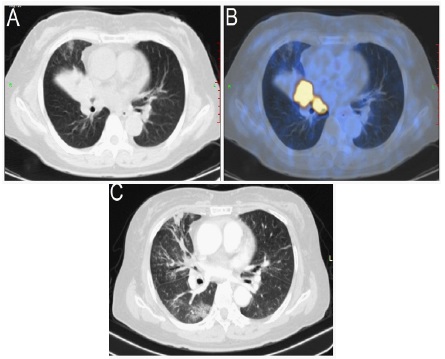
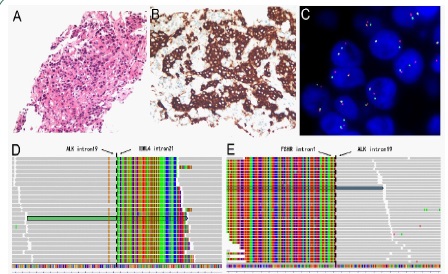
A 63-year-old nonsmoker female was admitted to the hospital with a complaint of persistent stimulating dry cough with hoarseness for 2 months. A Positron Emission TomographyComputed Tomography (PET-CT) scan revealed a 3.7 cm × 2.8 cm mass (SUVmax 20.2) in the right middle lobe adjacent to the hilum (Figure 1A & 1B) with multiple bone metastases and lymph node metastases. Lung biopsy confirmed the pathological diagnosis of poorly differentiated adenocarcinoma of the lung with a solid growth pattern (Figure 2A). Immunohistochemistry (IHC) demonstrated strong positivity for Thyroid Transcription Factor 1 (TTF-1) and Napsin A and negative positivity for cytokeratin 5/6 and P40. The examination of the ALK fusion by immunohistochemistry and Fluorescence In Situ Hybridization (FISH) was positive (Figure 2B & 2C). Finally, her disease was diagnosed as right lung adenocarcinoma (cT2N3M1c, stage IVB). The tumour tissue obtained from the biopsy was submitted for genomic testing by Next-Generation Sequencing (NGS) based on a pancancer 168-gene panel, as well as plasma cell-free DNA (circulating tumour DNA [ctDNA]). The NGS sequencing assay on the Illumina platform showed that the patient had a double ALK fusion: FSHR-ALK (F1:A20, 27.22% abundance in tissue, Figure 2D) and EML4-ALK (E21: A20, 32.09% abundance in tissue, Figure 2E). The FSHR gene and the ALK gene map to chromosome 2p with opposite orientations.
Alectinib was administered orally 300 mg twice daily beginning on September 18, 2020, and 1 month later, imaging re-examination revealed a significant decrease in tumour size and markedly relieved atelectasis (Figure 1C). According to the Response Evaluation Criteria in Solid Tumours (version 1.1), the patient was considered to have a partial response to alectinib. Unfortunately, the patient’s lung infection worsened.
Gender |
Age |
Pathological Pattern |
Fusion Type |
Treatment |
Follow-up |
|
1 14 |
male |
57 |
lung adenocarcinoma |
EML6-ALK, FBXO11-ALK |
chemotherapy,crizotinib |
partial response for 12 months |
2 13 |
male |
28 |
lung adenocarcinoma |
EML4-ALK, BCL11A-ALK |
crizotinib |
partial response for 13 months in lung,recurrent liver metastasis |
3 15 |
male |
44 |
lung adenocarcinoma |
EML4-ALK, PRKCB-ALK |
crizotinib |
partial response at 1 month, weak enlargement at 6 months |
4 16 |
male |
32 |
non-small cell lung cancer |
CCNY-ALK, ATIC-ALK |
crizotinib |
partial response for 6 months |
5 5 |
male |
60 |
lung adenocarcinoma |
EML4-ALK, BIRC6-ALK |
alectinib |
partial response for 3 months |
6 2 |
female |
55 |
lung adenocarcinoma |
EML4-ALK, CDK15-ALK |
crizotinib |
partial response for 23 months |
7 17 |
female |
64 |
lung adenocarcinoma |
EML4-ALK, NLRC4-ALK |
crizotinib |
stable disease for 10 months |
8 18 |
male |
44 |
lung adenocarcinoma |
DYSF-ALK, ITGAV-ALK |
chemotherapy,crizotinib |
partial response for 3 months in lung,brain metastases continued to grow |
current |
female |
63 |
lung adenocarcinoma |
EML4-ALK,FSHR-ALK |
alectinib |
partial response for 2 month |
Discussion
In this study, our patient is the first reported patient to harbour an EML4-ALK and FSHR-ALK double fusion and exhibit a remarkable response to alectinib. In 2007, SODA et al. identified the first case of an EML4-ALK fusion in non-small-cell lung cancer [10]. To date, more than 20 ALK fusion partners have been reported in NSCLC [5]. Because of the low occurrence rate of double ALK fusions, data on these fusions remain scarce. FSHR is a glycosylated transmembrane receptor protein that is overexpressedand is further related to an increase in tumour cell proliferation and aggressiveness. FSHR belongs to family 1 of the G protein-coupled receptors and is the receptor for follicle stimulating hormone and functions in gonad development. Among the detected fusion genes, intron 1 of the FSHR gene and intron 19 of the ALK gene were disrupted and ligated. Notably, a novel EML4-ALK variant (E21, A20) was identified, which has been previously reported in colorectal carcinoma [11]. In this report, the genes also mapped to chromosome 2p in opposite orientations. Intron 21 of the EML4 gene and intron 20 of the ALK gene were broken and rearranged. As far as we know, the FSHR-ALK fusion has been reported in only one patient, but the specific follow-up date remains unknown [12]. Although there is no direct evidence to support that the FSHR-ALK fusion is a driver mutation, considering the overexpression of the FSHRALK fusion in NSCLC samples, it is possible that the FSHR-ALK rearrangement may act as a driver mutation.
To date, cases of double ALK fusion are still rare, and few cases have been reported (Table 1). Baodong et al. reported a case with a double ALK fusion, EML4-ALK and BCL11A- ALK that exhibited a partial response to crizotinib for 13 months [13]. After crizotinib treatment, the abundance of BCL11A-ALK could not be measured through NGS of ctDNA, and only a low abundance of the EML4-ALK fusion was detected. Jiangming et al. described a patient with the double ALK fusion EML4-ALK and BIRC6-ALK who received alectinib treatment for 3 months and did not exhibit adverse drug reactions [5]. In addition, some reports also confirmed that patients with double ALK fusions may be sensitive to crizotinib [2,14-18]. However, not all lung adenocarcinomas with ALK fusion may benefit from treatment with ALK-TKIs. Mengnan et al. reported a case with a novel HIP1-ALK fusion variant (H19:A20) that exhibited primary resistance to crizotinib treatment [19]. Moreover, the PFS of patients with ALK-positive NSCLC treated with alectinib as a first-line treatment varies due to differences in the fusion forms of ALK [20]. Therefore, it is of considerable clinical significance to continuously study the form of ALK fusion and the correlation between ALK fusions and the sensitivity of patients to targeted drugs.
Conclusion
In summary, this report is the first to describe a lung adenocarcinoma patient who had novel double ALK fusion, EML4- ALK and FSHR-ALK, and was sensitive to alectinib. The curative effect of alectinib in the treatment of a lung adenocarcinoma patients with an EML4-ALK and FSHR-ALK double ALK fusion provides a valuable reference for other patients with such gene alterations. Furthermore, this case also indicates that dynamic NGS tests can facilitate clinical decision-making by detecting molecular changes.
Acknowledgements: The authors thank our patient and her family, as well as our colleagues at Wuhan union hosptial. Consent for publication has been obtained from the patient herself.
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81773022 and 82072333); Natural Science Foundation of Hubei Province (No. 2020CFB808).
Declaration of conflicting interests: The authors declare no competing interests.
Research ethics and patient consent: The present study was approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology (reference no. S-377) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016; 5: 288- 300.
- Guo J, Shi J, Yao M, Jin Y, Liu D, et al. A rare double ALK fusion variant EML4-ALK and CDK15-ALK in lung adenocarcinoma and response to crizotinib: A case report. Medicine (Baltimore). 2020; 99: e22631.
- Kawamura T, Murakami H, Kobayashi H, Nakashima K, Omori S, Wakuda K, et al. Leptomeningeal recurrence after long-term alectinib therapy for non-small cell lung cancer harboring an EML4-ALK fusion protein. Invest New Drugs. 2019; 37: 184-187.
- Feng T, Chen Z, Gu J, Wang Y, Zhang J and Min L. The clinical responses of TNIP2-ALK fusion variants to crizotinib in ALK-rearranged lung adenocarcinoma. Lung Cancer. 2019; 137: 19-22.
- Zhong JM, Zhang GF, Lin L, Li DY, Liu ZH. A novel EML4-ALK BIRC6-ALK double fusion variant in lung adenocarcinoma confers sensitivity to alectinib. Lung Cancer. 2020; 145: 211-212.
- Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363: 1693-1703.
- Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018; 19: 1654-1667.
- Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine. 2017; 377: 829- 838.
- Yoshida T, Oya Y, Tanaka K, Shimizu J, Horio Y, et al. Differential Crizotinib Response Duration Among ALK Fusion Variants in ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol. 2016; 34: 3383-3389.
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, et al. Identification of the transforming EML4-ALK fusion gene in nonsmall-cell lung cancer. Nature. 2007; 448: 561-566.
- Lin E, Li L, Guan Y, Soriano R, Rivers CS, Mohan S, et al. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and nonsmall cell lung cancers. Mol Cancer Res. 2009; 7: 1466-1476.
- Jin K, Zhang X, Chen H, Zhou Q and Yang J. Uncommon ALK fusion partners in advanced ALK-positive non-small-cell lung cancer. Journal of Clinical Oncology. 2018; 36: 8561-8561.
- Qin BD, Jiao XD, Liu K, Wu Y, Zang YS. Identification of a Novel EML4-ALK, BCL11A-ALK Double-Fusion Variant in Lung Adenocarcinoma Using Next-Generation Sequencing and Response to Crizotinib. J Thorac Oncol. 2019; 14: e115-e117.
- Lin H, Ren G, Liang X. A Novel EML6-ALK FBXO11-ALK Double Fusion Variant in Lung Adenocarcinoma and Response to Crizotinib. J Thorac Oncol. 2018; 13: e234-e236.
- Luo J, Gu D, Lu H, Liu S, Kong J. Coexistence of a Novel PRKCBALK, EML4-ALK Double-Fusion in a Lung Adenocarcinoma Patient and Response to Crizotinib. J Thorac Oncol. 2019; 14: e266- e268.
- Wu X, Zhou H, He Z, Zhang Z, Feng W, et al. Coexistence of a novel CCNY-ALK and ATIC-ALK double-fusion in one patient with ALK-positive NSCLC and response to crizotinib: A case report. Transl Lung Cancer Res. 2020; 9: 2494-2499.
- Wu X, Wang W, Zou B, Li Y, Yang X, Liu N, et al. Novel NLRC4-ALK and EML4-ALK double fusion mutations in a lung adenocarcinoma patient: A case report. Thorac Cancer. 2020; 11: 1695-1698.
- Yin J, Zhang Y, Zhang Y, Peng F and Lu Y. Reporting on Two Novel Fusions, DYSF-ALK and ITGAV-ALK, Coexisting in One Patient with Adenocarcinoma of Lung, Sensitive to Crizotinib. J Thorac Oncol. 2018; 13: e43-e45.
- Li M, Tang Q, Chen S, Wang Y. A novel HIP1-ALK fusion variant in lung adenocarcinoma showing resistance to Crizotinib. Lung Cancer. 2021; 151: 98-100.
- Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, et al. Updated Efficacy and Safety Data and Impact of the EML4- ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non-Small Cell Lung Cancer in the Global Phase III ALEX Study. J Thorac Oncol. 2019; 14: 1233-1243.