Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
An unruptured “Pocket – Like” giant apical pseudoaneurysm of the left ventricle: A case report
Rekik Bassem; Yaakoubi Wael*; Zouari Fourat; Boudiche Selim; Mghaith Fathia; Abdejlil Farhati; Elarbi Noureddine; Ouali Sana; Ben Hlima Manel; Mourali Mohamed Sami
Hopital La Rabta Adult Cardiology Service Tunis Ministry of Public Health, Tunisia.
*Corresponding Author: Yaakoubi Wael
Hopital La Rabta Adult Cardiology Service Tunis
Ministry Of Public Health, Tunisia.
Email: yaakoubiwael3@gmail.com
Received : Jun 02, 2021
Accepted : Jul 15, 2021
Published : Jul 20, 2021
Archived : www.jcimcr.org
Copyright : © wael Y (2021).
Abstract
Cardiac pseudoaneurysm is rare and appears mostly as tardive complication of Acute Myocardial Infarct (AMI). Its apical localization is also scarce as it is usually described in posterior or lateral wall of left ventricle. Its diagnosis is based on cardiac imaging. We report a case of a hypertensive, diabetic and smoking 64-year-old man with a past history of anterior AMI. He was symptomatic of chest discomfort. Physical examination indicates an apical murmur, his electrocardiogram showed a regular sinus rhythm and a complete left branch block. The Transthoracic (TTE) echocardiography revealed a giant apical pocketlike aneurysm lined with a clot. Cardiac Magnetic Resonance Imaging (CMRI) confirmed the diagnosis of an apical pseudoaneurysm due to ischemic heart disease in the stage of severe heart failure.
Citation: Bassem R, Wael Y, Fourat Z, Selim B, Fathia M. An unruptured “Pocket – Like” giant apical pseudoaneurysm of the left ventricle: A case report. J Clin Images Med Case Rep. 2021; 2(4): 1232.
Introduction
Left ventricular pseudoaneurysm is a contained rupture of ventricular wall by adherent pericardium or scar tissue, it is a rare serious complication that occurs following Acute Myocardial Infarction (AMI) and may lead to sudden death, ventricular arrhythmias, pump failure, or thromboembolism. It is usually located in the inferior and posterior basal walls and rarely in the apex, in contrast a true aneurysm is mostly located in the apicoanterior wall. Distinction of a pseudoaneurysm from a true aneurysm can be difficult but characteristic imaging features are usually effective in reaching a definitive diagnosis. Surgical repair is associated with an important risk of complications.
Case presentation
A 64-year-old diabetic hypertensive and smoking man presented to our hospital with chest pain at rest and frequent awakening due to chest discomfort at night for about 04 days. He had no palpitations, no syncope. There was no history of stroke or embolic event in the past.
He revealed a history of anterior AMI 3 years ago. Percutaneous Coronary Intervention (PCI) was performed at that time for the occluded left anterior descending artery with a Bare Metal Stent (BMS). Post-PCI echocardiography showed moderately impaired left ventricular function (Ejection Fraction about 40%) with akinesia of the apex and apical walls.
Physical examination at the admission indicated blood pressure of 150/90 mmHg, heart rate of 80 beats/min, and a continuous murmur at apical area.
Electrocardiography (ECG) showed a complete left branch block.
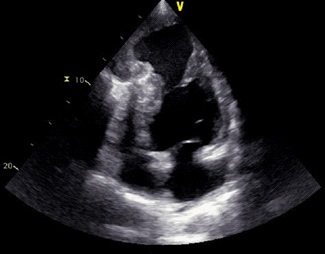
Transthoracic Echocardiography (TTE) showed a severe ventricular dysfunction with an ejection fraction less than 20%, a giant apical pocket-like aneurysm lined with a clot. There was minimal functional mitral insufficiency. The antero-lateral and infero-septal walls were hypokinetic. No pericardial effusion or right ventricular dysfunction were noted (Figure 1).
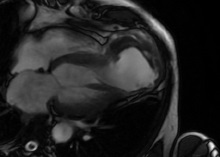
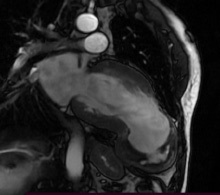
Cardiac Magnetic Resonance Imaging (CMRI) was performed to clarify the anatomy and aid the planning of surgical repair confirmed the presence of a large apical pseudoaneurysm (80 mm X 70 mm) of Left Ventricle (LV) and showed a clot which had a thickness about 30 mm. Ejection fraction was estimated by CMRI to be 18% when including the aneurysmal sac. No rupture signs were noted in this exam (Figure 4,5).
Discussion
Mechanical complications after MI are now much less frequent following implementation of effective early revascularization strategies.
Left ventricular pseudoaneurysm is a rare complication of acute myocardial infarction. It usually occurs with inferior and/ or posterior infarction.
Postinfarction pseudoaneurysms, in contrast to true aneurysms, are much more likely to rupture, causing hemopericardium and death.
The highest risk of rupture exists within the first 10 days to 3 months (mean of 18 weeks) after infarction [1].
The signs and symptoms in the population at risk (history of MI, prior cardiac surgery) are neither sensitive nor specific for pseudoaneurysm.
Therefore, imaging is usually required to diagnose, to identify the presence of other pathology accounting for the patient’s signs and symptoms, or to differentiate true LV aneurysm from pseudo-one which typically have a narrow neck in contrast [2].
Although angiography had historically been recommended as the imaging modality of choice for cardiac pseudoaneurysms, advances in non-invasive imaging during the past decade have improved the ability to accurately identify this condition [3].
The most accurate non-invasive diagnostic method has been echocardiography [4].
Initial evaluation with transthoracic echocardiography may be revealing but in suspected cases, angiography, and cardiac Magnetic Resonance Imaging (MRI) will have a higher diagnostic yield as coronary angiography shows the need for concomitant bypass grafting. CMRI has high spatial resolution and the ability to characterize tissue, thus enabling non-invasive identification of the pericardium and the presence of thrombi, and can distinguish between necrotic and normal myocardium, which is not always possible with other imaging modalities [5].
Besides providing information on overall morphology and function, particularly ventricular volumes and systolic and valve function, CMRI provides better morphological definition of a pseudoaneurysm’s location, extension and its relations to adjacent structures. Moreover, delayed enhancement sequences enable accurate assessment of the location and extent of the infarcted area and of viable myocardium, thus contributing to pre-operative planning [6].
In view of its growing availability and advances in acquisition sequences, the technique is increasingly used in clinical practice, as in the case presented. It has added diagnostic value over echocardiography, particularly in patients with poor image quality, as well as to determine the relations of the pseudoaneurysm to the mitral valve and papillary muscles.
The myocardial infarction is the most common cause of left ventricular pseudoaneurysms, also prior aortic valvular surgery and endocarditis most often precede pseudoaneurysms at the mitral-aortic intervalvular fibrosa [3].
The reason for the detection of fewer anterior LV pseudoaneurysms may be due to the fact that an anterior LV wall rupture terminates in rapid hemopericardium, tamponade, shock, and death; whereas, posteriorly it tends to get contained by the pericardium and inflammatory adhesions forming a pseudoaneurysm rather than cardiac tamponade [7].
After diagnosis, no guidelines address whether to pursue follow-up imaging. For select patients managed non-surgically, follow-up imaging to assess for expansion may be reasonable if it could affect management [8].
In a series of 52 patients with ventricular pseudoaneurysm followed up for a median of 4 years, 42 patients underwent surgery, but notably 10 patients managed non-surgically did not have any occurrence of rupture [9].
A case similar to ours was recently published by Mohamed Farag and als. in February 2019 reporting an pseudo-anvrysm (constituted within 02 months post AMI) which was about 90mm treated surgically [10].
Conclusion
As well the pseudoaneurysm as its apical location are rare. Although often challenging to diagnose, advances in noninvasive imaging (MRI and Echography) improve the ability to distinguish it.
References
- Epstein JI, Hutchins GM. Subepicardial aneurysms: A rare complication of myocardial infarction. Am J Med. 1983; 75: 639‑44.
- Antman EM. Treatment of ST elevation myocardial infarction. In: Libby P, Braunwald E, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, Pa: Saunders/Elsevier. 2012.
- Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol. 1998; 32: 557-561.
- Roelandt JR, Sutherland GR, Yoshida K, Yoshikawa J. Improved diagnosis and characterization of left ventricular pseudoaneurysm by Doppler color flow imaging. J Am Coll Cardiol. 1988; 12: 807-811.
- Harrity P, Subramanian R, Patel A, Blanco J. Improved diagnosis and characterization of postinfarction left ventricular pseudoaneurysm by cardiac magnetic resonance imaging. Clin Cardiol. 1991; 14: 603‑606.
- Konen E, Merchant N, Gutierrez C, Provost Y, Mickleborough L, Paul NS, et al. True versus False Left Ventricular Aneurysm: Differentiation with MR Imaging—Initial Experience. Radiology. 2005; 236: 65-75.
- Bisoyi S, Dash A, Nayak D, Sahoo S, Mohapatra R. Left ventricular pseudoaneurysm versus aneurysm a diagnosis dilemma. Ann Card Anaesth. 2016; 19: 169.
- Hulten EA, Blankstein R. Pseudoaneurysms of the Heart. Circulation. 2012; 125: 1920‑1925.
- Yeo TC. Clinical Profile and Outcome in 52 Patients with Cardiac Pseudoaneurysm. Ann Intern Med. 1998; 128: 299.
- Farag M, Lota A, Rosendahl U, Roussin I. Large left ventricular apical pseudoaneurysm: A multimodal imaging approach guiding successful diagnosis and surgical management. Gati S, Izgi C, Bonaros N, éditeurs. Eur Heart J - Case Rep. 2019; 3.