Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
Burned-out testicular tumor: Two case reports
Castillo C1; Camejo N1; Centurion D2; Carzoglio J2; Cepellini R3; Krygier G1
1 Oncology Department, Hospital de Clínicas Dr. Manuel Quintela, Montevideo, Uruguay.
2 Pathology Department, Hospital de Clinicas Dr. Manuel Quintela, Montevideo, Uruguay.
3 Urology Department, Hospital de Clínicas, Dr. Manuel Quintela, Montevideo, Uruguay.
*Corresponding Author: Cecilia Castillo
Oncology Department, Hospital de Clínicas Dr. Manuel
Quintela, Av Italia s/n, CP 11600, Montevideo, Uruguay.
Email: cascecilia@gmail.com
Received : Aug 30, 2021
Accepted : Sep 30, 2021
Published : Oct 07, 2021
Archived : www.jcimcr.org
Copyright : © Castillo C (2021).
Abstract
Spontaneous regression of a testicular tumor or burned-out testicular tumor is a rare phenomenon in patients with testicular germ cell tumors. The condition is characterized by tumor metastases, suspicious findings of the testicular tumor on ultrasound imaging, and partial or total histological regression of the primary testicular tumor after orchiectomy without treatment. Clinically, patients present with a disseminated tumor with symptoms related to the metastatic site without a palpable testicular tumor. Patients may also present with elevated levels of tumor biomarkers, depending on the histology. The etiopathogenesis of this phenomenon remains unclear and may involve immunologic factors as well as necrosis due to tumor growth beyond the available blood supply.
We report here two clinical cases of patients treated at our center, both presenting with symptoms caused by retroperitoneal lymph node dissemination but different histologiesas well as a different clinical course.
Keywords: germ cell tumor; testicular cancer; spontaneous regression.
Abbreviations: AFP: Alpha-Fetoprotein; BEP: Bleomycin, Etoposide, And Cisplatin; BHCG: Beta-Human Chorionic Gonadotropin; DNA: Deoxyribonucleic Acid; LDH: Lactate Dehydrogenase; PCT: Polychemotherapy; PLAP: Placental Alkaline Phosphatase; VEIP: Vinblastine, Ifosfamide and Cisplatin; WHO: World Health Organization.
Citation: Castillo C, Oviedo Y, Camejo N, Centurion D, Carzoglio J, et al. Burned-out testicular tumor: Two case reports. J Clin Images Med Case Rep. 2021; 2(5): 1348.
Case I
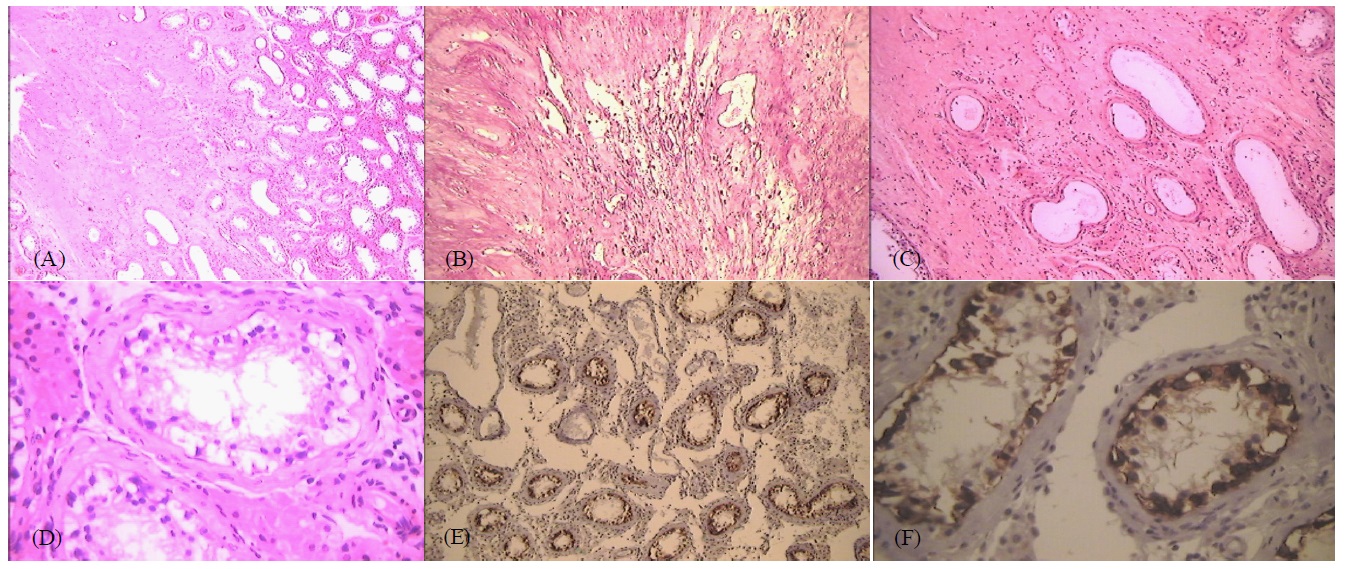
A 25-year-old patient visited the emergency room for upper gastrointestinal bleeding and a 20-day history of progressive melena, showing clinical anemia and a thick central abdominal tumor without a palpable testicular tumor. Upper gastrointestinal endoscopy revealed extrinsic compression of the inferior duodenum, with active bleeding and without evidence of its origin, for which blind hemostasis was performed. A chest, abdominal and pelvic tomography showed a retroperitoneal mass measuring 160 X 120 X 130 mm, involving the large vessels and displacing the gastroduodenal region, without other abnormal tomographic findings. A scrotal ultrasound performed for the suspected retroperitoneal mass of probable testicular origin showed a 11 mm hypoechoic lesion of the lower pole of the right testicle. Elevated levels of the tumor markers alpha-fetoprotein (AFP: 13.671 mU/mL) and beta-human chorionic gonadotropin (BHCG: 5.318 mU/mL) confirmed the diagnosis of a germ cell tumor. A right radical orchiectomy was performed and the histological study showed a whitish lesion in the right testicle, with irregular edges and a diameter of 8 mm, located less than 1 cm from the albuginea, consisting of a fibro-hyalinized area trapping the remnants of hyalinized tubules and surrounded by siderophages in hematoxylin and eosin (H&E)-stained preparations. The adjacent testicular parenchyma showed testicular germinal atrophy with peritubular fibrosis (Figure 1A and 1B). Some seminiferous tubules showed scarce germ cells presenting anisocaryosis and discrete nuclear hyperchromatism, modifications attributable to tubular atrophy (Figure 1C and 1D). The rest of the testicular parenchyma had a preserved structure, with diffuse Leydig (interstitial) cell hyperplasia.
The analysis was complemented with immunohistochemical studies being the positivity for PLAP in germ cells of the seminiferous tubules the most significant finding (Figure 1E and 1F). Elevated tumor marker levels were found after orchiectomy. The patiente underwent chemotherapy (CT) treatment with BEP (cisplatin, etoposide, and bleomycin) every 21 days for four cycles. At the end of treatment, the levels of both tumor markers had normalized but a residual periaortic retroperitoneal mass measuring 4 cm in diameter was present. A salvage retro peritoneal lymphadenectomy was recommended; however, the mass was unresectable during surgery due to its adherence to vascular structures. The anatomopathological study of the incisional biopsy was compatible with a diagnosis of a mature teratoma. During follow-up, approximately 1 month after surgery, the levels of both tumor markers were elevated, with no other evidence of dissemination on imaging studies. A second-line CT treatment with VeIP (vinblastine, ifosfamide, and cisplatin) was repeated every 21 days for four cycles, which also resulted in the normalization of the levels of both markers and persistence of the retroperitoneal tumor mass. Mass resection was re-attempted, but complete resection was not possible as the mass remained adhered to vascular structures. The anatomopathological study identified a lymph node structure associated with fibrous connective tissue with the presence of two nodules of mature cartilage, which were interpreted in this context as compatible with a cartilaginous remnant of a mature teratoma.
Considering this situation, a reoperation was proposed to the patient for complete resection of the retroperitoneal mass at an international reference center. The patient refused and was lost to follow-up; he died 2 years later from complications of a pulmonary embolism.
Case II
A 32-year-old patient, presented with sudden-onset pain in his right lumbar fossa, radiating to the flank. Ultrasound of the urinary and testicular system showed a an abdominal and scrotal ultrasound was performed, which showed the presence of a hypoechoic adenopathic conglomerate in the retroperitoneum at the intercavoaortic and lateroaortic levels, without clear vascularization on Doppler. A solid hypoechoic lesion was observed in the upper pole of the right testis, which was well defined, with discrete vascularization on color Doppler, and measuring 9 X 5 X 8 mm. The lesion was associated with diffusely distributed microcalcifications in the right testicular parenchyma. The patient had no palpable testicular tumor.
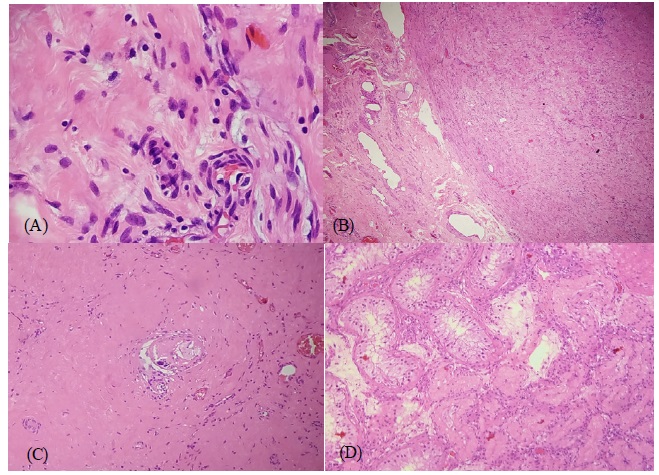
Chest-abdominal and pelvic tomography showed a retroperitoneal conglomerate with heterogeneous contrast and a hypodense center with necrosis, measuring 52 x 35 x 45 mm. Measurement of tumor biomarker levels owing to the suspicion of retroperitoneal metastasis of a testicular germ cell tumor showed normal levels (lactate dehydrogenase [LDH] 483 u/L; AFP 1.63 mU/mL; BHCG 1.02 mU/mL). A right radical orchiectomy revealed a whitish tumor measuring 11 X 11 X 13 mm, with a hyalinized fibrosclerotic area with mild lympho-monocyte permeability and ‘trapped’ collapsed tubules (Figure 2A, 2B and 2C). Other areas of the testis had tubules with thickened and hyalinized basement membranes, atrophy, and no spermatogenesis (Figure 2D). The examined areas showed no signs of malignancy.
A tomography-guided biopsy of the retroperitoneal adenopathic conglomerate revealed a seminomatous germ cell tumor. Treatment with CT was proposed; as the patient had no children, sperm was cryopreserved before treatment initiation. BEP was repeated every 21 days for three cycles. Tomography performed at the end of chemotherapy treatment showed a residual retroperitoneal tumor with a diameter of 7 mm. Imaging follow-up was subsequently performed.
Discussion
The concept of spontaneous regression of testicular tumors arose from autopsy studies in patients who died from disseminated germ cell tumors with normal testicular examination findings. This type of tumor was first described by Prym in 1927 in a patient with a visceral and retroperitoneal disseminated extragonadal choriocarcinoma who died without a primary tumor and had scar tissue in the testis at autopsy [2]. Slater et al was the first to refer to this phenomenon as a ‘burn out primary tumor’ in 1955 [3].
Since then, despite their infrequent presentation, multiple cases have been published in the international literature, including isolated cases and small series of patients.
This phenomenon was first recognized by the World Health Organization (WHO) in 2016 in the chapter on testicular and para-testicular tissue tumors in ‘Tumors of the Male Urinary and Genital System’ [4].
Spontaneous regression has also been described in malignant melanoma, renal cancer, breast cancer and lymphoma, and is the most extensively studied phenomenon in malignant melanoma, where up to 50% of primary tumors may show partial spontaneous regression of primary tumors [5,6,7].
The cause of regression of the primary testicular lesion is unknown, although cell necrosis secondary to ischemia or, more likely, an immunological mechanism, has been postulated as its origin. The ischemia hypothesis is based on a reduction in blood flow due to the high proliferation rate caused by tumor growth and/or intermittent testicular torsion [8,9].
The immunological hypothesis is based on an immune response mediated by cytotoxic T lymphocytes that recognize tumor antigens and destroy neoplastic cells, triggering subsequent fibrosis. This hypothesis was based on the spontaneous regression of melanoma in which an inflammatory reaction was observed with an increased lymphocyte count and interleukin 2 receptor expression.
In these cases, a dense infiltrate of T lymphocytes is later replaced by fibrosis [9]. One of the hypotheses proposed for the regression of malignant melanoma is that repeated exposure to common tumor antigens may trigger cell-mediated immunity reactions [11].
The anatomopathological analysis of samples obtained from orchiectomy in these two patients showed both macroscopic and microscopic evidence consistent with the diagnostic criteria proposed in the literature.
The macroscopic analysis of testes with partial or total tumor regression shows the appearance of indurated, whitish, fibrous lesions with a scar-like appearance in the form of nodules (single or multiple), bands, lines, or stars [4,12-15].
The microscopic criteria for this phenomenon were defined by Mostofi in 1973 based on the analysis of 17 patients with testicular cancer with regression, with characteristic findings as follows [16].
-Dense or lax acellular collagenous fibrous scar with hyalinized seminiferous tubules, hemosiderin deposits, and scattered plasma cells.
-Hematoxylin deposits separated by basement membrane and scar tissue, consisting of calcium and Deoxyribonucleic acid (DNA), resulting from tubular cell necrosis.
Tumor regression can be complete or partial. The most frequent finding is intratubular neoplasia, although there may be teratomatous elements and, less frequently, foci of residual invasive tumors [12,17,18].
Only the first case in the present series showed a ‘Minimal’ intratubular germ cell neoplasia in seminiferous tubes adjacent to the fibrous focus in the immunohistochemical study. Although intratubular neoplasia was not observed, PLAP labeled the seminiferous tube cells, indicating that they corresponded to modified germ cells.
More recently, in 2016, the WHO defined lymphoplasmacytic inflammatory infiltrate (present in around 90% of cases), tubular hyalinization (approximately 70%), increased vascularity (50%), hemosiderophages (44%), and thick intratubular calcifications as the diagnostic criteria for testicular tumor regression, in addition to seminiferous tubule atrophy and sclerosis (100%), in situ germ cell neoplasia (approximately 50%), Leydig cell hyperplasia (45%), and intratubular microliths (30%) with respect to the periphery. Moreover, in situ germ cell neoplasia and large and thick calcifications, which are believed to derive from intratubular growth, necrosis, and/or calcification of the embryonal carcinoma, are specific findings of this phenomenon.
Thus, pathologists confronted with a testicular scar, even if it is not accompanied by the aforementioned criteria, should consider the possibility of a spontaneously regressing testicular tumor [4,12,18].
Regarding the histological subtype of the tumor and its potential for spontaneous regression, although choriocarcinoma was historically the subtype most frequently associated with this phenomenon, recent reports more often observe seminoma, while teratoma remains the least likely subtype to regress [17,19].
The first case in this series was a mixed non-seminomatous tumor, although we could not exclude a seminomatous component. Since the AFP levels were >100 mU/mL, the tumor was thought to be associated with a yolk sac tumor component; moreover, as the BHCG was >5,000 mU/mL, it was thought to be also associated with a choriocarcinoma and/or embryonal carcinoma component. In the second case, the lymph node biopsy and the normal levels of tumor biomarkers did not suggest a classic seminoma.
The symptoms of this phenomenon are similar to those of any tumor mass. The retroperitoneal mass of the first case caused extrinsic compression on the duodenum; probable infiltration of the walls likely explained the bleeding observed by endoscopy, while the second case presented with pain in the lumbar fossa, reflecting the presence of an extensive retroperitoneal disease with probable involvement of the psoas muscle or nerve roots.
In patients with extragonadal germ cell tumors with normal testicular examination findings, the question is whether the tumor is a primary extragonadal germ cell tumor (a rare entity, corresponding to 3-5% of germ cell neoplasms) or if it is a nonpalpable testicular tumor in the early stage or if it has spontaneously regressed, being the latter more likely for retroperitoneal germ cell tumors than for those located in the mediastinum and central nervous system [20,21].
As also observed in both patients in the present case series, testicular palpation findings are usually normal. Since this procedure is not very accurate, a high-resolution scrotal ultrasound is necessary to identify the primary tumor or its indirect signs.
Testicular tumors that have regressed show non-specific ultrasound manifestations. The most frequent findings are nodular or linear, hyper- or hypoechogenic lesions, signs of testicular atrophy, and/or shadows that reflect calcifications or fibrosis [22-24].
When the ultrasound is inconclusive, scrotal magnetic resonance imaging may be useful to better define indeterminate findings [25].
Both patients in our series presented hypoechoic nodular lesions on scrotal ultrasound, which was associated with microlithiasis in one case. Radical orchiectomy was necessary after diagnosis of the burned-out testicular tumor. This procedure confirms the diagnosis and has therapeutic purposes as it eradicates the primary tumor focus, since the persistence of intratubular neoplastic germ cells increases the risk of disease recurrence. In addition, the blood-testicular barrier prevents the passage of cytotoxic agents to the seminiferous tubules [1,19,26].
The extratesticular disease was treated with cisplatin-based PCT (BEP regimen), similar to that used for later-stage testicular tumors. These tumors are staged and treated similarly to conventional testicular germ cell tumors, since there are no guidelines to specifically evaluate this phenomenon given its low frequency, although it remains unknown whether their prognosis is similar to that for testicular tumors or whether the regression could impact the prognosis of these patients [17].
The first case corresponded to a stage IIIC (pT0 N3 MO S3) non-seminomatous tumor with a bulky retroperitoneal mass measuring 16 cm in diameter. The pronounced elevation of AFP to the S3 level (>10,000 ng/mL) placed it into the high-risk category according to the International Germ Cell Tumor Collaborative Group Classification. These patients have a >50% chance of a lasting response to PCT, and up to 50% of patients die of the disease [26,27].
Our patient presented a partial response to the first-line treatment with BEP and normalization of tumor marker levels, with a residual mass measuring 4 cm. In non-seminomatous tumors, retroperitoneal lymphadenectomy with complete surgical resection of residual retroperitoneal disease ≥1 cm is standard of care for teratoma and/or viable tumor resection [28]. The probabilities that residual retroperitoneal masses with normal post-computed tomography and tumor marker levels are fibrosis/necrosis, teratoma, and a viable tumor are 40–50%, 35–40%, and approximately 10%, respectively, with higher probabilities of a viable tumor or teratoma with increasing residual tumor size [29].
The aorta, inferior vena cava, and renal vessels may adhere to retroperitoneal tumors, as was observed in our patient and which prevented a complete resection. Thus, surgeons must be trained in vascular techniques and these surgeries should be performed in specialized centers [30,31,32]. In our opinion, the best way that would have improved the patient’s prognosis would have been to adequately integrate the systemic CT and surgery with complete resection of the residual mass.
The second case corresponded to a stage IIC (pT0N3 M0) seminomatous tumor, hence the indication for cisplatin-based PCT with the BEP regimen every 21 days for three cycles, which resulted in a very good response, with a residual retroperitoneal tumor measuring <1 cm, which will be monitored by followup imaging.
It is common for patients with stage II seminomas to evidence residual disease on imaging after CT treatment, which occurs in up to 80% of those who present with bulky disease [33].
Many of these residual masses do not contain viable tumor and disappear during follow-up in approximately half of the patients, with a low probability of presenting a viable tumor when the residual tumor is <3 cm, as is the case in the second patient in our series [34]. As for the prognosis, the second case would correspond to an advanced seminoma in the low-risk category according to the International Germ Cell Tumor Collaborative Group Classification, with a 5-year overall survival rate (OSR) of 92% [35]. LDH has recently been shown to be a prognostic factor in advanced low-risk seminoma, in which patients with LDH levels <2.5 times the upper normal limit, as in the case of our patient, have a better prognosis (3-year OSR of 97% versus 92% with LDH above this value) [36].
Conclusion
Burned-out testicular cancer is a rare phenomenon; case reports and small series published over several decades have contributed to its recognition by the WHO in 2016. The condition is characterized by metastases from a germ cell tumor, without a palpable testicular tumor, with an image suspicious of tumor on scrotal ultrasound and a partial or total histological regression of the primary testicular tumor without treatment after orchiectomy. Based on histological examinations, the most frequent cases are of seminoma and the etiopathogenesis appears to mainly involve immunological events. Diagnosis is confirmed by orchiectomy, with characteristic elements, and eradicates the primary tumor focus, since, otherwise, the persistence of intratubular neoplastic germ cells could increase the likelihood of disease recurrence. Due to its low frequency, it is unknown whether the clinical behavior of the disease is similar to that in patients with testicular tumors or whether regression affects their prognosis. Regardless, these tumors should be staged and treated like conventional testicular germ cell tumors.
Declaration: Written informed consent was obtained from the patient's next of kin (for case 1) and the patient (for case 2) for publication of these case reports.
Disclosure: The author reports no conflicts of interest in this work.
References
- Castillo C, Krygier G, Carzoglio J, et al. Gastrointestinal bleeding as the first manifestation of a burned-out tumour of the testis. Clin Transl Oncol. 2005; 7: 458-63.
- Prym P. Spontanheilung eines bösartigen wahrscheinlich chorionephiteliornatösen Gewächses im Hoden. Virchows Arch Pathol Anat. 1927; 265: 239-258.
- Slater G, Schultz H, Kreutzmann W. Occult testicular tumor. J Am Med Assoc. 1955; 157: 911–912.
- Ulbright T, Amin M, Balzer B, et al. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th. Lyon: IARC. Tumours of the testis and Paratesticular tissue. 2016; 185–258.
- Salman T. Spontaneous tumor regression. J Oncol Sci. 2016; 2: 1–4
- Shai A, Avinoach I, Sagi A. Metastatic malignant melanoma with spontaneous and complete regression of the primary lesion. Case report and review of the literature. J Dermatol Surg Oncol. 1994; 20: 342-5.
- Cervinkova M, Kucerova P, Cizkova J. Spontaneous regression of malignant melanoma - is it based on the interplay between host immune system and melanoma antigens? Anticancer Drugs. 2017; 28: 819-830.
- Simpson AH, Calvert DG, Codling BW. The shrinking seminoma. JR Soc Med. 1990; 83: 187.
- Naseem S, Azzopardi A, Shrotri N, Mufti GR. The shrinking seminoma--fact or fiction? Urol Int. 2000; 65: 208-10.
- Halliday GM, Patel A, Hunt MJ, Tefany FJ, Barnetson RSC. Spontaneous regression of human melanoma/nonmelanoma skin cancer: Association with infiltrating CD4+ T cells. World J. Surg. 1995; 19: 352–358.
- Saleh FH, Crotty Ka, Hersey P et al. Autonomous histopathological regression of primary tumors associated with specific immune responses to cancer antigens. J Pathol. 2003; 200: 383-95.
- Balzer B, Ulbright T. Spontaneous regression of testicular germ cell tumors: an analysis of 42 cases. Am J Surg Pathol. 2006; 30: 858–86.
- McCarthy WA, Cox BL, Laucirica R, Moss JE. Fine Needle Aspiration Diagnosis of a Metastatic Mixed Germ Cell Tumor from a “Burned Out” Testicular Primary with Florid Leydig Cell Hyperplasia. Ann Clin Cytol Pathol. 2015; 1: 1013
- Parada D, Peña K,Moreira O, et al. Extragonadal retroperitoneal germ cell tumor: primary versus metastases? Arch Esp Urol. 2007; 60: 713–719.
- Nakazaki H, Tokuyasu H, Takemoto Y, et al. Pulmonary metastatic choriocarcinoma from a burned-out testicular tumor Intern Med. 2016; 55: 1481–148.
- Mostofi FK, Price EB. Tumors of the Male Genital System. Washington, DC: Armed Forces Institute of Pathology. 1973.
- Astigueta JC, Abad-Licham MA, Agreda FM, Leiva BA, De la Cruz JL. Spontaneous testicular tumor regression: Case report and historical review. Ecancermedicalscience. 2018; 12: 888.
- Williamson SR, Delahunt B, Magi-Galluzzi C, et al. The World Health Organization 2016 classification of testicular germ cell tumours: A review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology. 2017; 70: 335-346.
- Almeida M, Amaral L, Viveiros D et al. Paraduodenal mass as initial presentation of burned-out testicular tumour: Case report and literature review. Journal of Surgical Case Reports. 2020; (3): 1–4.
- Ojea A, Rodríguez A, Pérez D et al. Tumor extragonadal de células germinales con fenómeno “burned out”. Actas Urol Esp. 1999; 23: 880-884.
- Busch J, Seidel C, Zengerling F. Male Extragonadal Germ Cell Tumors of the Adult. Oncol Res Treat. 2016; 39: 140-4
- Tasu J, Faye N, Eschwegw P, et al. Imaging of burned-out testis tumor: five new cases and review of the literature JUltrasound Med. 2003; 22: 515–521.
- De Luis Pastor E, Villanueva Marcos A, Zudaire Díaz-Tejeiro B et al. Ecografía escrotal: perlas, patrones y errores [Scrotal ultrasound: pearls, patterns and pitfalls]. Actas Urol Esp. 2007; 31: 895-910.
- Peroux E, Thome A, Geffroy Y, et al Burned-out tumour; Retroperitoneal metastases; Ultrasound Diagn Interv Imaging. 2012; 93: 796–798.
- El Sanharawi I, Correas JM, Glas L, Ferlicot S, Izard V, et al. Non-palpable incidentally found testicular tumors: Differentiation between benign, malignant, and burned-out tumors using dynamic contrast-enhanced MRI. Eur J Radiol. 2016; 85: 2072- 2082.
- International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997; 15: 594-603.
- van Dijk MR, Steyerberg EW, Habbema JD. Survival of non-seminomatous germ cell cancer patients according to the IGCC classification: An update based on meta-analysis. Eur J Cancer. 2006; 42: 820-6.
- Daneshmand S, Albers P, Fosså SD et al. Contemporary management of postchemotherapy testis cancer. Eur Urol. 2012; 62: 867-76.
- Pfister D, Busch J, Winter C et al. Pathohistological findings in patients with nonseminomatous germ cell tumors (NSGCT) who undergo postchemotherapy retroperitoneal lymph node dissection (PC-RPLND) for small tumours. J Clin Oncol 2011; 29: 244a.
- Heidenreich A, Haidl F, Paffenholz, et al. Surgical management of complex residual masses following systemic chemotherapy for metastatic testicular germ cell tumours. Ann Oncol. 2017; 28: 362-367.
- Riggs SB, Burgess EF, Gaston K et al. Postchemotherapy surgery for germ cell tumors--what have we learned in 35 years? Oncologist. 2014; 19: 498-506.
- Christmas TJ, Smith GL, Kooner R Vascular interventions during post-chemotherapy retroperitoneal lymph-node dissection for metastatic testis cancer. Eur JSurg Oncol. 1988; 24: 292-7.
- Peckham MJ, Horwich A, Hendry WF. Advanced seminoma: treatment with cis-platinum-based combination chemotherapy or carboplatin (JM8). Br J Cancer. 1985; 52: 7-13.
- Flechon A, Bompas E, Biron P, Droz JP. Management of postchemotherapy residual masses in advanced seminoma. J Urol. 2002; 168: 1975-9.
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997; 15: 594-603.
- Beyer J, Collette L, Sauvé N et al International Germ Cell Cancer Classification Update Consortium. Survival and New Prognosticators in Metastatic Seminoma: Results From the IGCCCG-Update Consortium. J Clin Oncol. 2021; 39: 1553-1562.