Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
Desmoplastic fibroma of bone: Amorphological and immunehistochemical characterization
Gabriel Cao1,2*; Graciela Ottaviano2 ; AnalíaFusaro1 ; Julián Mendez1 ; Daniel Navacchia1
1División Anatomía Patológica, Hospital General de Niños “Dr. Pedro de Elizalde”, Buenos Aires, Argentina.
2 Universidad Abierta Interamericana-CONICET. Centro de Altos Estudiosen Ciencias Humanasy de la Salud, Buenos Aires, Argentina.
*Corresponding Author: Gabriel Cao
División Anatomía Patológica, Hospital General de
Niños “Dr. Pedro de Elizalde”, Avenida Montes de Oca
40 (1270AAN), Buenos Aires, Argentina.
Email: gabrielcao@fibertel.com.ar
Received : Oct 04, 2021
Accepted : Nov 22, 2021
Published : Nov 29, 2021
Archived : www.jcimcr.org
Copyright : © Cao G (2021).
Abstract
Background: Desmoplastic Fibroma (DF) of bone is a locally aggressive and infrequent benign neoplasm. Recently was described a role of vascular endothelial growth factor in the interstitial fibrotic processes.
Case presentation: A 13-year-old female presented with pain, swelling and limitation of movements in right forearm. An osteolytic lesion at the distal end of the right radius was shown, with pathologic concentration of Technetium 99 and slight enhancement of soft tissue lesion employing computerized axial tomography. The surgical biopsy showed nodular formations of hyalinized collagen fibers arranged in thick bands with few well-differentiated interstitial fibroblasts / myofibroblasts, focallyexpressing VEGF-A.
Conclusion: The intramedullary neoplastic proliferation is limited by the cortical bone, provoking compression of the intratumorally micro-vessels, favoring both, the extracellular matrix and VEGF-A synthesis. Future research should include therapeutic intervention with anti-CD117 and anti-VEGF-A drugs, with the aim of limiting tumor growth, facilitating the complete surgical excision of the neoplasm.
Keywords: desmoplastic fibroma; vascular endothelial growth factor; hyalinization; neoplasm progression.
Citation: Sackey AD, Sackey AD, John B, Lee-Duah RO. A look into “Outlining the challenges of Covid-19 health crises in Africa’s maritime industry: The case of maritime operations in marine warranty surveying practice”: Discussing ‘three pillar challenge’. J Clin Images Med Case Rep. 2021; 2(6): 1429.
Introduction
Desmoplastic fibroma (DF) of bone is an infrequent and locally aggressive benign neoplasm, representing 0.06% of all bone neoplasms and 0.3% of benign neoplasms in this location [1]. In pediatric population, the occurrence of this entity is lowest than 1% of bone neoplasms. The entity was described in 75% of males’ cases, being the lower jaw (22%), femur (15%), pelvic bones (13%), radius (12%) and tibia (9%) the predominant locations [2,3]. Radiologically presents a circumscribed lytic lesion, without peripheral sclerosis or periosteal reaction [4,5]. Microscopically, the entity is described as a proliferation of spindle or stellar cells, with little cytological atypia and abundant stromal collagen [6]. On the other hand, arole of vascular endothelial growth factor (VEGF) in the interstitial fibrosis processes was recently described [7].
Due to the unusual nature of this entity in pediatric population, our objective was describing the histopathology findings, emphasizing the immunohistochemical expression for VEGF-A and its relationship with fibro-sclerotic process.
Report of the case
A 13-year-old female presented with pain, swelling, limitation of prono-supination movements in right forearm and a pre vious traumatic history in this area. Radiologically an osteolytic lesion at the distal end of the right radius was shown, with a septum formation inside it, and zonal disruption of the cortical bone, producing a mass effect on the neighboring soft tissues, deforming the cortical bone of the ulna. Pathologic concentration of Technetium 99 (Tm99) was observed at scintigraphy. The intravenous contrast reveled a slight enhancement of soft tissue lesion employing computerized axial tomography (CAT).
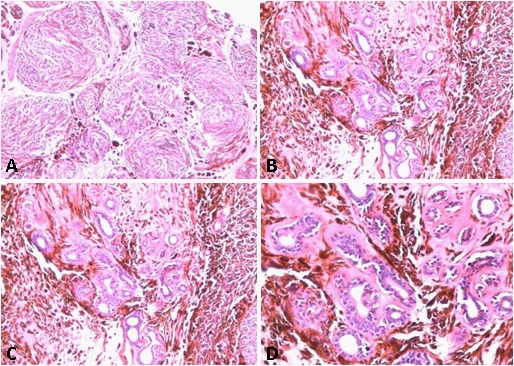
A biopsy was performed, getting multiple irregular whitish fragments, that together measured 3 X 2 X 0.5 cm and weighed 3 grams. The tissue was routinely processed and then colored with H&E and Masson’s Trichrome (TM) techniques. In the histological sections, bone trabeculae were observed in contact with nodular formations of dense and hyalinized collagen fibers arranged in thick bands with few well-differentiated interstitial fibroblasts (Figure 1A). This histological finding, together with the characteristic radiological images of the lesion were consistent with the desmoplastic fibroma diagnosis. Subsequently, an osteotomy was performed with osteosynthesis, and bone graft obtained from the right iliac crest, applied to the distal end of the ipsilateral radius, together the resection of the neighboring soft tissues. A lobulated whitish formation was received of 12 X 6.5 X 5.5 cm and 180 grams, with a hard-elastic consistency, whitish color, and swirling appearance. The histological sections showed similar characteristics than previously described in the initial biopsy, made up of an intraosseous component seated in the medullary space of the radius bone, which demonstrated marked stromal desmoplasia, accompanied by few fibroblastic cells with mild anisokaryosis. This lesion transgressed the bone cortex, spreading towards the adjacent soft tissues (Figure 1B), where showed wide sectors with little collagenization and numerous cells with, likewise, fibroblastic appearance, mild anisokaryosis, dispersed chromatin, conspicuous nucleoli, and isolated typical mitoses (less than 1 per 10 high-power fields). Interestingly, the stroma was focally lax, which allowed the identification of cells with broad and stellate cytoplasm, vesicular nuclei and conspicuous nucleolus, characteristics that gave them the appearance of active myofibroblasts (Figure 1F). The cell population was organized in short interlocking fascicles, eventually vorticial (Figure 1A). These sectors underwent transition with others that showed a gradual increase of stromal collagen, until reaching areas with keloid-like appearance (Figure 1C and D). In the periphery, the neoplasm demonstrated entrapment of adipose tissue and skeletal muscle.
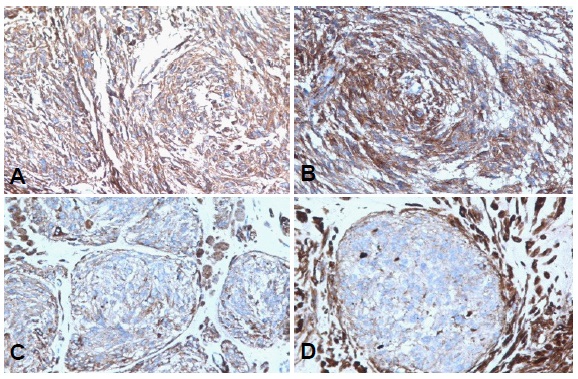
Immunohistochemical techniques were performed with an automated equipment to analyze the expression of Vimentin, actin HHF35, α-Smooth Muscle Actin (α-SMA), Desmin, VEGF-A, CD34, CD117 and Ki67. The lesion was diffuse and strongly positive for Vimentin and focally for HHF35 actin, α-SMA (Figure 2, A and B respectively), Desmin (Figure C), and VEGF-A (Figure 2D). In this sense, the cell population expressed a higher proportion of α-SMA and VEGF-A, specially involving those with a fibroblastic / myofibroblastic appearance. In contrast, immunolabelling for Desmin and HHF35 actin were observed only in few cell groups. CD34 showed positivity in vascular endothelium, while Ki67 (Figure 2E) was expressed in less than 5% of the neoplastic cell nuclei, especially nearto destroyedbone cortex. Finally, CD117 (Figure 2F) was expressed in occasional focal groups of round and medium-sized cells, following a membrane pattern like that observed in pluripotent hematopoietic cells.
The authors confirm that they have parental consent to publish the information presented in this paper. The importance of communicating the findings obtained was emphasized to improve the pathogenetic understanding of the entity and the best therapeutic approach to this infrequent pathology
Discussion
DF is an infrequent entity, which has traditionally been associated with microscopically similar extra-abdominal desmoid tumors [8]. In the diagnostic differentiation, it is important to determine the precise location of both processes [9]. A common histological component is the presence of hyalinized or sclerosing collagenous matrix, with low cell density in these areas, being a characteristic observed in various benign and malignant processes. There is evidence that supports the complex interrelation between neoplastic cells and the surrounding extracellular matrix (ECM), which promotes the transdifferentiation of fibroblasts to myofibroblasts, the degradation and new synthesis of extracellular components (remodeling), angiogenesis, proliferation, and expansion of neoplastic cells [10]. In addition, focal groups of CD117 positive cells were observed, describing a membranous immunohistochemical pattern, indicative of stem cells, probably of bone marrow origin, suggesting an alternative source of fibroblasts and myofibroblast, beyond the transdifferentiation phenomenon. CD117 encodes a membrane receptor tyrosine kinase that is expressed in gastrointestinal stromal tumors (GISTs) but notin smooth muscle or neural tumors of the gastrointestinaltract. Mutations in c-kit leading to the constitutive activation of thetyrosine kinase are believed to have a role in the tumorigenesis of the overwhelming majority of GISTs. There have been several reports of KIT immunostainingin a limited number of soft tissue tumors other than GISTs, including metastatic melanoma, clear cell sarcoma, angiosarcoma, extraskeletal Ewing sarcoma, and desmoid fibromatosis, at times with conflicting results. In this sense, CD117 positive cells could be facilitate increase of extracellular hyalinized matrix and, indirectly, the VEGF-A production. Whether ckit inhibitors might provide therapeutic benefit to the subset of patients with desmoplastic fibroma whose tumors show immunoreactivity for c-kit remains to be determined [11]. On the other hand, in our case, we also observe an increased extracellular matrix, that included hyalinized collagenous matrixspecially at medullary space of bone. The hyaline collagen fibers were surrounded by activated myofibroblasts, which expressed α-SMA. Also, these areas showed lower vascularity by the reduction of CD34 immunostaining compared to sectors expanded beyond the bone. The fibroblasts/myofibroblasts cells were increased in sites of cortical bone rupture, with extension towards the soft tissue’s neighbors. These sectors showed the highest cellular density of the neoplasia, where myofibroblasts and endothelial cells showed nuclear immunolabelling for Ki67. Likewise, the extracellular matrix was reduced, without evidence of hyalinized collagen fibers. Remodeling of the ECM plays a key role in neoplastic growth and invasion. In this sophisticated interplay, the fibroblastsare the main contributors to ECM changes [12]. VEGF is a subfamily of growth factors, which are important signalingproteins involved in both vasculogenesis and angiogenesis.Today, is accepted that VEGF-A participate in angiogenic and fibroticprocess [13,14]. A recent work indicate that VEGFA promote liver fibrosis progression via inducing the VEGF-A/ VEGFR-2 signaling pathway-mediated crosstalk between hepatocytes and sinusoidal endothelial cells [15]. Also, VEGF-A together with TGF-β1, contributing with the submesothelial fibrosis and neoangiogenesis observed in patients with peritoneal dialysis [16]. In a similar neoplasm to DF, such as the extraabdominal desmoid tumor (DT), overexpression of VEGF plays a key role in tumor progression and recurrence [17]. However, in the presented case, we observed groups of fusiform cells expressing VEGF-A not associated to sclerosing matrix. Also, this situationcoexists to increased expression of Ki67 in vascular endothelial cells nuclei. In the other hand, a differential point between DT and DF is the pathogenic overexpression of VEGF in DT. In base of our case, this postulate is not exact [18].
In liver diseases, the hypoxia is a condition that link between the fibrogenic, angiogenic and carcinogenic phenomena [19]. For this reason, we hypothesize, at least in this case, that the initial intramedullary neoplastic proliferationis limited by the cortical bone, provokingcompression of the intratumorallymicro-vessels in certain zones, decreasing the blood and oxygen supply. This situation favoring the expansion of ECM by accumulation of interstitial hyalinizing collagen, probably due the participation of the transcription factor hypoxia inducible factor-1 (HIF-1), activating the system fibroblast / myofibroblast and increasing the collagen deposition [20]. Then, the mechanical collapse of the tumoralvessels, promotes VEGF-Asynthesis, angiogenesis, and neoplastic expansion, especially in areas of cortical bone destruction and extension on extraosseous soft tissue.
Finally, we accept the limitation of this hypothesis, however, we believe that it`s a starting point for future research that includes therapeutic intervention with anti-CD117 or anti-VEGF-A drugs, with the aim of limiting tumor growth, facilitating complete surgical excision of this aggressive neoplasm.
Declarations
Acknowledgements: We want specially to thank to Beatriz Uribe and Lara Dahir for his qualified technical support.
Disclosure statement: The authors declare that there is no conflict of interest regarding the publication of this manuscript.
References
- Gong YB, Qu LM, Qi X, Liu JG. Desmoplastic fibroma in the proximal femur: A case report with long-term follow-up. Oncol Lett. 2015; 10: 2465-67.
- Said-Al-Naief N, Fernandes R, Louis P, Bell W, Siegal GP. Desmoplastic fibroma of the jaw: a case report and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101: 82-94.
- Levrini G, Pattacini P. Desmoplastic fibroma of the distal tibia: A case report of a minimally invasive histological diagnosis. Mol Clin Oncol. 2016; 5: 537-539.
- Crim JR, Gold RH, Mirra JM, Eckardt JJ, Bassett LW. Desmoplastic fibroma of bone: radiographic analysis. Radiology. 1989; 172: 827-32.
- Stevens J, Moin S, Salter D, Patton JT. Desmoplastic Fibroma: A Rare Pathological Midshaft Femoral Fracture Treated with Resection, Acute Shortening, and Re-lengthening: A Case Report. JBJS Case Connect. 2019; 9: e0022.
- Evans S, Ramasamy A, Jeys L, Grimer R. Desmoplastic fibroma of bone: A rare bone tumor. J Bone Oncol. 2014; 3: 77-9.
- Abbas O, Mahalingam M. Desmoplasia: not always a nothing. Histopathology. 2011; 58: 643-59.
- Hardy R, Lehrer H. Desmoplastic fibroma vs. desmoid tumor of bone. Two cases illustrating a problem in differential diagnosis and classification. Radiology. 1967; 88: 899- 901.
- Rabin D, Ang LC, Megyesi J, Lee DH, Duggal N. Desmoplastic fibroma of the cranium: case report and review of the literature. Neurosurgery. 2003; 52: 950-4.
- Qu Z, Van Ginkel S, Roy AM, Westbrook L, Nasrin M, Maxuitenko Y et al. Vascular endothelial growth factor reduces tamoxifen efficacy and promotes metastatic colonization and desmoplasia in breast tumors. Cancer Res 2008; 68: 6232-6240.
- Jason L Hornick, Christopher D M Fletcher. Immunohistochemical staining for KIT (CD117) in soft tissue sarcomas is very limited in distribution. Am J Clin Pathol. 2002; 117: 188-193.
- Najafi M, Farhood B, Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem 2019; 120: 2782-2790.
- Karvinen H, Pasanen E, Rissanen TT, Korpisalo P, Vähäkangas E, Jazwa A et al. Long-term VEGF-A expression promotes aberrant angiogenesis and fibrosis in skeletal muscle. Gene Ther. 2011; 18: 1166-1172.
- Jin HY, Lee KS, Jin SM, Lee YC. Vascular endothelial growth factor correlates with matrix metalloproteinase-9 in the pleural effusion. Respir Med. 2004; 98: 115-122.
- Zhaoyong Yan, Kai Qu, Jing Zhang, Qichao Huang, Ping Qu, Xinsen Xu et al. CD147 promotes liver fibrosis progression via VEGF-A/VEGFR2 signalling-mediated cross-talk between hepatocytes and sinusoidal endothelial cells. Clin Sci (Lond). 2015; 129: 699-710.
- Tetsuyoshi Kariya, Hayato Nishimura, Masashi Mizuno, Yasuhiro Suzuki, Yoshihisa Matsukawa, Fumiko Sakata et al. TGF-β1-VEGF-A pathway induces neoangiogenesis with peritoneal fibrosis in patients undergoing peritoneal dialysis. Am J Physiol Renal Physiol. 2018; 314: F167-F180.
- Matono H, Tamiya S, Yokoyama R, Saito T, Iwamoto Y, Tsuneyoshi M, Oda Y. Abnormalities of the Wnt/β-catenin signalling pathway induce tumour progression in sporadic desmoid tumours: correlation between β-catenin widespread nuclear expression and VEGF overexpression. Histopathology. 2011; 59(3): 368-375.
- Mills BG, Frausto A, Brien E. Cytokines associated with the pathophysiology of aggressive fibromatosis. J Orthop Res. 2000; 18: 655-662.
- RosmorducO, Housset C. Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis. 2010; 30: 258-270.
- Darby IA, Hewitson TD. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 2016; 365: 553-562.