Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
Mammary analogue secretory carcinoma (MASC): A literature review and report of two cases
Yi Hai Li*; Naveed Basheeth; Aneesh Kumar
Department of Otorhinolaryngology, Palmerston North Hospital, Midcentral District Health Board, Palmerston North, New Zealand.
*Corresponding Author: Yi Hai Li
Palmerston North Hospital, 50 Ruahine Street, Roslyn,
Palmerston North, 4442 New Zealand.
Email: yh.felix.li@gmail.com
Received : Oct 11, 2021
Accepted : Nov 25, 2021
Published : Dec 02, 2021
Archived : www.jcimcr.org
Copyright : © Li YH (2021).
Abstract
Introduction: Mammary analogue secretory carcinoma (MASC) is a rare salivary gland tumour. It is generally a low-grade lesion that presents as a slow growing painless swelling with an indolent course with good survival. Diagnosis is typically via the highly specific ETV6- NTKR3 gene fusion with the translocation t(12;15)(q13;q25).
Method: A literature review was conducted through PubMed, Cochrane, and Medline. We also present two cases.
Results: Although typically regarded as a low-grade lesion, highgrade features, such as nodal metastases, more solid and trabecular architecture with necrosis, and the ETV6-X fusion gene, have been described. Our cases were diagnosed through morphologic and immunohistochemical features despite absence of the classic fusion gene.
Conclusion: MASC can be diagnosed in absence of the highly specific ETV6-NTKR3 in presence of certain morphologic and immunohistochemical features. Although it is a typically low-grade lesion, several high-grade features have been described that may require further treatment.
Keywords: head and neck neoplasms; salivary gland; parotid gland; salivary gland neoplasms; parotid neoplasms; immunohistochemistry; mammary analogue secretory carcinoma.
Citation: Li YH, Basheeth N, Kumar A. Mammary analogue secretory carcinoma (MASC): A literature review and report of two cases. J Clin Images Med Case Rep. 2021; 2(6): 1446.
Introduction
Mammary Analogue Secretory Carcinoma (MASC) is a rare, relatively recently described salivary gland tumour, with the first being reported by Skalova et al. in 2010 [1]. Since then, some 300 cases have been published in the literature.
Epidemiology
The true incidence is unknown due to rarity and previous misclassification. Luk et al. found 9 MASC cases in a review of 190 malignant salivary gland tumours (4.5%), whereas Majewska et al. found 7 MASC cases in a review of 183 cases (4%) in a similar study [2,3].
Clinical presentation
It most commonly occurs in the parotid gland, but it can also arise from minor salivary glands in the lips, hard palate, base of tongue, as well as submandibular gland [4-6].
It is a low-grade tumour that typically presents as a slowgrowing, painless swelling, though high-grade lesions presenting with pain and facial nerve paresis have been reported [4,5,7,8]. It occasionally has extracapsular extension, perineural invasion, and cervical nodal metastasis, whilst distant metastasis is rare [4,9]. Local recurrence is uncommon [5]. Despite it usually behaving indolently, it can also behave more aggressively, however there are no definitive prognostic factors to help differentiate this currently. Treatment is typically by gland extirpation, with or without neck dissection, and sometimes radiation therapy. However, given the rarity and lack of prognosticating factors, there are no consensus regarding treatment and follow up [4].
Histopathology
In WHO 2017 4th Edition Classification of Salivary Tumours, in addition to introducing the concept of high-grade transformation and modification of salivary carcinomas including polymorphous and cribriform adenocarcinoma, clear cell carcinoma, intraductal carcinoma, there are three new entities that have been added including MASC, sclerosing polycystic adenosis and intercalated duct lesions [10].
MASC shares the morphology and genetics of secretory carcinoma of the breast, a subtype of infiltrating ductal carcinoma, which is equally rare [1]. Previously it has been labelled as acinic cell carcinoma, mucoepidermoid carcinoma, and adenocarcinoma, not otherwise specified [11,12]. It typically occurs in young male patients, with a mean age of 46 years old [4]. However, paediatric cases have been reported [13,14]. The defining characteristic is the ETV6-NTRK3 fusion and translocation: t(12;15) (q13;q25), which can both be identified on FISH or PCR [1]. This translocation has also been identified in infantile fibrosarcoma and congenitalmesoblastic nephroma [15]. Cases without classical ETV6 gene rearrangement may harbour alternative gene rearrangements (“ETV6-X fusion”) that may be associated with more aggressive clinical course of the disease [16].
Grossly it is a well-circumscribed, unencapsulated multilobulated mass, subdivided into smaller segments by fibrous septa. Microscopically the tumour cells have uniform round and vesicular nuclei with central nucleoli and eosinophilic pink vacuolated cytoplasm. Cells are arranged into microcystic, cribriform, tubular, papillary, follicular or solid nests. Microscopic foci of invasion are often present, but the overall mitotic activity is low. High grade MASC variations are characterised by a more solid and trabecular architecture with necrosis, diminished secretions, larger cells and more prominent nucleoli with atypia [6].
Intraluminal secretions may be seen in the microcystic or tubular structures. Such material is usually mucicarmine, periodic acid-Schiff (PAS), and Alcian blue positive. It also stains with antibodies to epithelial membrane antigen (EMA). The microscopic features of MASC can be also recognized cytologically by fineneedle aspiration biopsy. Cells are positive for a wide-spectrum of cytokeratins (e.g. AE1/AE3, Cam5.2), low-molecular weight cytokeratins (CK7, CK8, CK19), vimentin, S-100, mammaglobin, GCDFP-15, MUC1, GATA-binding protein 3, adipophilin, α-amylase, DOG-1, SOX-10, and p63 [6].
Here we present two cases of MASC involving the parotid gland that were managed in our centre.
Case I
A 35-year-old man presented with one year of painless firm and mobile left parotid lump of about 2 cm which has not changed in size. There were no cranial nerve deficits on exam. He had a background of obesity, gout, asthma, depression, nonsmoker, and previous excess alcohol and methamphetamine intake. He is of New Zealand Maori background.
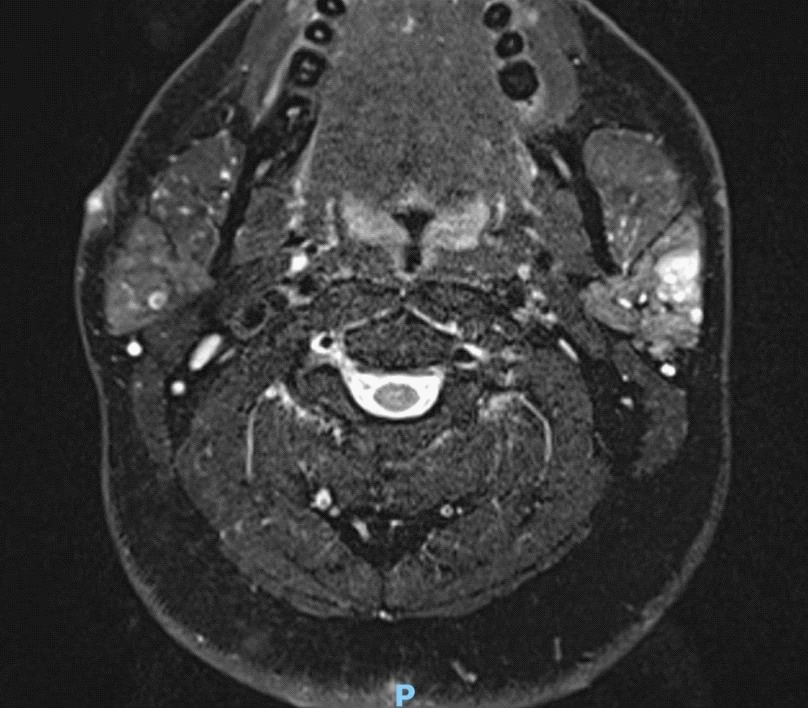
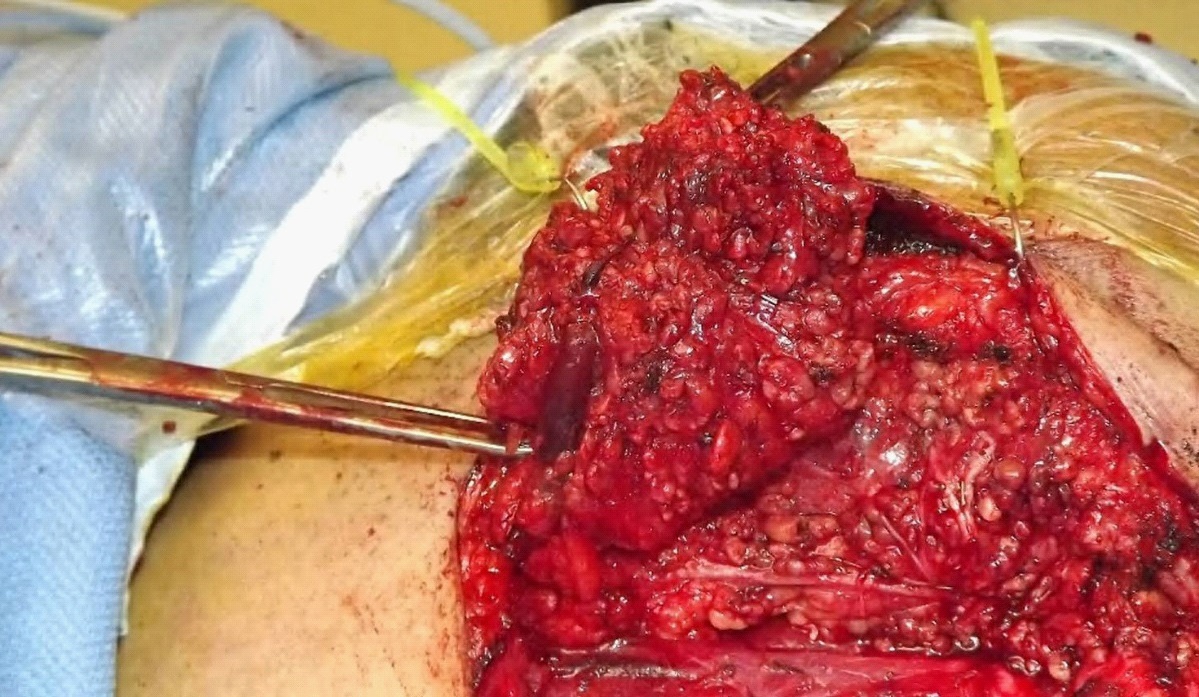
An MRI showed a superficial lobe tumour, with no pathological neck nodes (Figure 1). Subsequent core biopsy suggested a secretory carcinoma although it was ETV6 negative on FISH. The patient proceeded to superficial parotidectomy with preservation of facial nerve and without neck dissection (Figure 2).
The surgical specimen showed a 25 X 15 X 15 mm well-circumscribed but unencapsulated lesion with soft creamy yellow areas and firmer pale areas. The lesion was completely excised with closest margin of 3 mm and no involved lymph nodes or perineural invasion. Microscopically the tumour had lobules of cells with solid and microcystic growth pattern, with lobules separated by fibrous septa. There were also associated infiltrates of plasma cells and lymphocytes. The tumour cells contained small round nuclei with occasional mitoses (mitotic rate 1 in 10 high power fields). No PAS positive zymogen granules were identified.
FISH was repeated but was negative for ETV6 re-arrangement. A diagnosis of MASC was made based on morphology, presence of S100, Mammoglobin, GCDFP15, and negative DOG1 (Table 1). Multidisciplinary consensus favoured clinical observation without post-operative radiotherapy. There was no evidence of recurrence at 12 months following his operation.
Table 1:Immunohistochemistry (IHC) features of cases.
IHC |
Case 1 |
Case 2 |
S100 |
+ |
+ |
Mammoglobin |
+ |
+ |
CK5/6 |
- |
+ |
SOX10 |
- |
+ |
GCDFP15 |
+ |
- |
DOG1 |
- |
- |
BOB1 |
- |
- |
Mel A |
- |
- |
P40 |
- |
- |
MUC1 |
+ |
- |
CK8/18 |
+ |
+ |
CK19 |
+ |
+ |
Vimetin |
+ |
+ |
SMA |
- |
- |
GATA3 |
- |
- |
CK7 |
+ |
+ |
Case II
A 65-year-old man presented with one year of painless 3 cm firm left parotid lump with limited mobility, reportedly to be gradually enlarging. He is a non-smoker or drinker of alcohol and is otherwise well. He is of New Zealand Maori background.
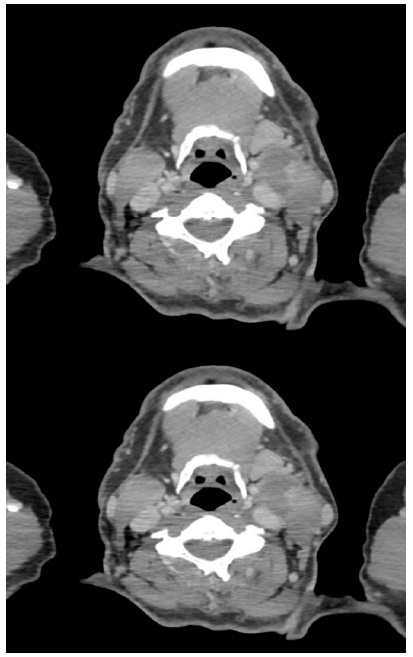
An MRI and CT showed a large 60 x 38 x 37 mm mass in left parotid, involving the deep lobe, extending into parapharyngeal space with mild compression of the carotid artery, but no lymph node involvement (Figure 3). He proceeded to core biopsy which was suggestive for secretory carcinoma, with FISH negative for ETV6 re-arrangement.
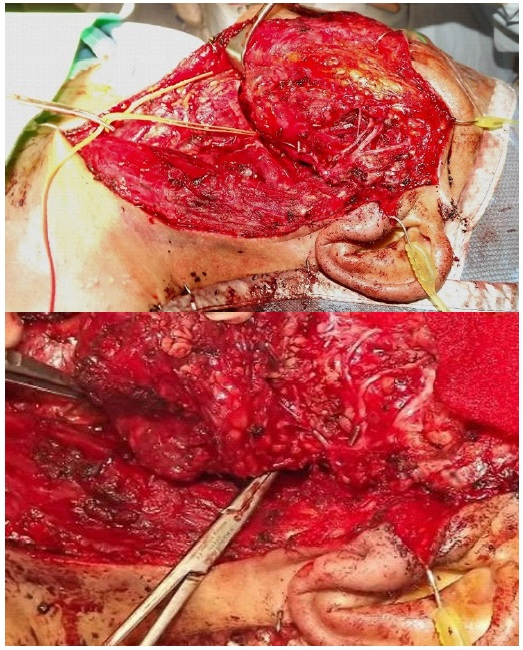
He proceeded to total conservative parotidectomy, parapharyngeal mass excision and selective neck dissection (Figure 4).
Final histology showed a completely excised, encapsulated parotid tumour 80 X 27 X 25 mm with solid and cystic components. Microscopically there are solid, nested, as well as infiltrative cord-like architecture. Cytologically there were cells with eosinophilia as well as focally vacuolated cytoplasm. Nuclei are uniform with occasional small but prominent nucleoli. No mitotic activity was seen. There was no perineural invasion and no involved lymph nodes. Immunohistochemical results are shown in Table 1.
Discussion
These cases demonstrate a typical presentation of MASC with a painless parotid swelling. The original core biopsies were suggestive of MASC, despite both not having the classic ETV6 rearrangement, which is specific to MASC in salivary cancers. As described in literature, a diagnosis of MASC can be made without the classic ETV6 fusion gene providing there is sufficient cytologic and immunohistochemical features [17] (Table 2).
Table 2:Typical features of mammary analogue secretory carcinoma (MASC) and its main differentials.
|
MASC |
AciCC |
ANOS |
Low-Grade ME |
Clinical |
Mainly parotid |
Mainly parotid |
Mainly minor salivary, followed by parotid |
50% major salivary |
Morphologic/ |
Microcysts, papillary proliferation, cribriform pattern. |
Solid, microcystic, papillary-cystic, follicular. |
Infiltrative. |
Cystic pattern |
IHC |
Strongly positive for Mammoglobin& S-100 STAT5 + |
PAS positive zymogen granules |
Mucin |
Diffuse p63 in squamoid cells |
Cytogenetic |
ETV6-NTRK3 |
Nil |
Nil |
CRTC1 (MECT1)-MAML2 |
These features are lobulated growth pattern, homogenous eosinophilic secretions, no PAS positive secretory zymogen granules, expression of S-100, mammoglobin, and vimetin [17,18]. Nevertheless, one must be mindful that mammoglobin reactivity can be found in a small proportion of low-grade cribriform cystadenoma, polymorphous low-grade adenocarcinoma, mucoepidermoid carcinomas, and pleomorphic adenomas, hence they are differentials in diagnosis of MASC [19]. Furthermore, co-reactivity of mammoglobin and S-100 were found in polymorphous low-grade adenosarcoma and adenoid cystic carcinoma [20,21]. As such, the diagnosis of MASC should not be made based on mammoglobin and S-100 reactivity alone, despite their high sensitivity.
An important differential diagnosis, both in terms of indolent behaviour and similar histological features is acinic cell carcinoma. It has been reported by Bishop et al. that a significant number of salivary acinic cell carcinomas have been reclassified as MASC [11]. The key difference is that acinic cell does not have the ETV6 gene rearrangement. It also typically has basophilic staining cytoplasm with PAS-positive zymogen granules instead of eosinophilic staining [4,6]. However, zymogen-poor acinic cell carcinomas do exist, though it is many of these that has been reclassified into MASC on subsequent review [11].
Although the pathological features of MASC have been well described, management is still controversial given lack of follow up data. Case 2 had a larger, more extensive lesion, however both did not have lymph node metastasis, and both had parotidectomies with preservation of facial nerve, without adjuvant radiotherapy. The mean disease-free survival has been reported to be 92 months, which was not statistically different to acinic cell carcinoma of 121 months [22]. As such, the management could be the similar, with surgical excision without radiotherapy in absence of clinical cervical nodal metastases. In presence of high-grade changes, namely nodal metastases which can occur in up to 25% of cases, more solid and trabecular architecture with necrosis, diminished secretions, larger cells and more prominent nucleoli with atypia, and ETV6-X fusion gene, then neck dissection and/or post-operative radiotherapy could be considered [8,20].
As a fore mentioned, FISH was negative for the classic ETV6 gene rearrangement in our cases, which could mean an alternative gene rearrangement may exist. Such a gene may be associated with more aggressive disease behaviour as described by Bissinger et al, however further studies with larger cohorts are needed to validate this association [16,23]. Both our cases had low-grade features and were thus managed without adjuvant radiotherapy.
Conclusion
It has been ten years since MASC was first described by Skalova. Our understanding of its pathological features has improved, with ETV6 being diagnostic, but acknowledging the utility of various morphologic and immunohistochemical features that would be supportive of MASC in absence of ETV6 gene rearrangement, which helped identify our two cases. Although the clinical presentation has largely remained consistent with the initial description of a low-grade lesion, its management is still uncertain. More studies are needed to elucidate prognostic features. Given the good survival reported to date, it would seem reasonable to manage as other low-grade salivary gland cancers.
Declarations
Acknowledgement: Dr Cynric Temple-Camp for assistance with pathology.
Ethical considerations: This case report follows local research guidelines and does not require IRB approval. Patient information has been anonymised. Clinical photographs and imaging for inclusion in publication has been utilised with consent by patients (signed consents available upon request).
Financial disclosure: none to disclose for all authors
Conflict of Interest: none to disclose for all authors
Key points
1. MASC is a recently described rare, low-grade, salivary gland cancer.
2. Diagnosis can be made without the classic ETV6-NTKR3 fusion
gene.
3. Co-reactivity of Mammoglobin and S-100 is sensitive but not
specific for MASC.
4. Potential high-grade features, including ETV6-X fusion gene, exist, but further studies are needed to validate this association.
5. Management is typically via extirpation of lesion with or without
neck dissection and adjuvant radiation therapy.
References
- Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J SurgPathol. 2010; 34(5): 599-608.
- Luk PP, Selinger CI, Eviston TJ, Lum T, Yu B, O’Toole SA, et al. Mammary analogue secretory carcinoma: an evaluation of its clinicopathological and genetic characteristics. Pathology. 2015; 47(7): 659-666.
- Majewska H, Skálová A, Stodulski D, Klimková A, Steiner P, Stankiewicz C, et al. Mammary analogue secretory carcinoma of salivary glands: A new entity associated with ETV6 gene rearrangement. Vir chows Arch. 2015; 466(3): 245-254.
- Sethi R, Kozin E, Remenschneider A, Meier J, VanderLaan P, Faquin W, et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope. 2014; 124(1): 188-195.
- Luo W, Lindley SW, Lindley PH, Krempl GA, Seethala RR, Fung KM. Mammary analog secretory carcinoma of salivary gland with high-grade histology arising in hard palate, report of a case and review of literature. Int J Clin Exp Pathol. 2014; 7(12): 9008- 22.
- Stevens TM, Parekh V. Mammary Analogue Secretory Carcinoma. Arch Pathol Lab Med. 2016; 140(9): 997-1001.
- Jung MJ, Song JS, Kim SY, Nam SY, Roh JL, Choi SH, et al. Finding and characterizing mammary analogue secretory carcinoma of the salivary gland. Korean J Pathol. 2013; 47: 36–43.
- Skalova A, Vanecek T, Majewska H, Laco J, Grossmann P, Simpson RH, et al. Mammary analogue secretory carcinoma of salivary glands with high-grade transformation: report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, beta-catenin, EGFR, and CCND1 genes. Am J SurgPathol. 2014; 38: 23–33.
- Connor A, Perez-Ordonez B, Shago M, Skalova A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J SurgPathol. 2012; 36: 27–34.
- Xu B, Katabi N. Evolving concepts and new entities in the 2017 WHO classification of salivary gland tumours. Diagn Histopathol. 2018; 24(5): 172-179.
- Bishop JA, Yonescu R, Batista D, Eisele DW, Westra WH. Most nonparotid ‘acinic cell carcinomas’ represent mammary analog secretory carcinomas. Am J SurgPathol. 2013; 37(7): 1053-1057.
- Chiosea SI, Griffith C, Assaad A, Seethala RR. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology. 2012; 61(3): 387-394.
- Rastatter JC, Jatana KR, Jennings LJ, Melin-Aldana H. Mammary analogue secretory carcinoma of the parotid gland in a pediatric patient. Otolaryngol Head Neck Surg. 2012; 146(3): 514-5.
- Hwang MJ, Wu PR, Chen CM, Chen CY, Chen CJ. A rare malignancy of the parotid gland in a 13-year-old Taiwanese boy: case report of a mammary analogue secretory carcinoma of the salivary gland with molecular study. Med Mol Morphol. 2014; 47(1): 57-61.
- Knezevich SR, Garnett MJ, Pysher TJ, Beckwith JB, Grundy PE, Sorensen PH. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. 1998; 58: 5046–5048.
- Bissinger O, Götz C, Kolk A, Bier HA, Agaimy A, Frenzel H, et al. Mammary analogue secretory carcinoma of salivary glands: diagnostic pitfall with distinct immunohistochemical profile and molecular features. Rare Tumors. 2017; 9(3):7162.
- Shah AA, Wenig BM, LeGallo RD, Mills SE, Stelow EB. Morphology in conjunction with immunohistochemistry is sufficient for the diagnosis of mammary analogue secretory carcinoma. Head Neck Pathol. 2015; 9(1): 85-95.
- Skalova A. Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morphologic and immunohistochemical spectrum of recently described entity. Head Neck Pathol. 2013; 7 Suppl 1(Suppl 1): S30-S36.
- Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH. Utility of mammaglobin immunohistochemistry as a proxy marker for the ETV6-NTRK3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol. 2013; 44(10): 1982-1988
- Skalova A, Bell D, Bishop JA, Inagaki H, Seethala R, Vielh P. Secretory carcinoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors, WHO Classification of Head and Neck Tumours, 4th ed. Lyon: IARC; 2017. p. 177-8.
- Patel KR, Solomon IH, El-Mofty SK, Lewis JS Jr, Chernock RD. Mammaglobin and S-100 immunoreactivity in salivary gland carcinomas other than mammary analogue secretory carcinoma. Hum Pathol. 2013; 44(11): 2501-2508.
- Chiosea SI, Griffith C, Assaad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J SurgPathol. 2012; 36(3): 343-50.
- Skálová A, Vanecek T, Simpson RH, Laco J, Majewska H, Baneckova M, et al. Mammary analogue secretory carcinoma of salivary glands: molecular analysis of 25 ETV6 gene rearranged tumors with lack of detection of classical ETV6-NTRK3 fusion transcript by standard RT-PCR: report of 4 cases harboring ETV6-X gene fusion. Am J SurgPathol. 2016; 40(1): 3-13.