Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
Histopathology of patient’s cyanide toxicity with brugada ECG pattern
Dang Viet Duc1*; Nguyen Tat Tho2; Pham Van Chinh1; Nguyen Thanh Huy1; Luu Quang Minh1; Pham Son Lam1; Pham Thai Giang1; C Anwar A Chahal3
1 Cardiovascular Intensive Care Unit, Heart Institute, 108 Military Central Hospital, Ha Noi, Vietnam.
2 Military Institute Forensic Medicine, Ha Noi, Vietnam.
3 University of Pennsylvania, Philadelphia, Pennsylvania, USA.
*Corresponding Author: Dang Viet Duc
Member of HRS, Cardiovascular Intensive Care Unit,
Heart Institute, 108 Military Central Hospital, No1 Tran
Hung Dao, Ha Noi, Vietnam.
Email: dangvietduc108@gmail.com
Received : Oct 18, 2021
Accepted : Dec 07, 2021
Published : Dec 14, 2021
Archived : www.jcimcr.org
Copyright : © Duc DV (2021).
Abstract
Cyanide is a potent and lethal poison which uncouples oxidative phosphorylation in mitochondria. We describe a case due to massive cyanide poisoning resulting in a type 1 Brugada ECG pattern, with full resolution as cyanide levels decreased and histopathology
Keywords: Brugada syndrome; cyanide; poisoning; histopathology; endocardium; epicardium.
Abbreviations: ACLS: Advanced Cardiac Life Support; BrS: Brugada Syndrome; CRRT: Continuous Renal Replacement Therapy; CPR: Cardiopulmonary Resuscitation; CN: Cyanide; ECG: Electrocardiogram; PEA: Pulseless Electrical Activity; ROSC: Return Of Spontaneous Circulation; RVOT: Right Ventricular Outflow Tract; VF: Ventricular fibrillation.
Citation: Duc D, Tho N, Chinh P, Huy N, Minh L, et al. Histopathology of patient’s cyanide toxicity with Brugada ECG Pattern. J Clin Images Med Case Rep. 2021; 2(6): 1474.
History of presentation
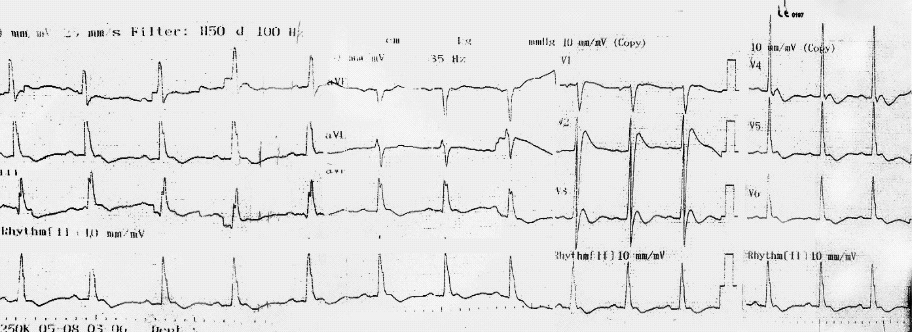
A 44-years-old male with no significant past medical history. We didn’t know he drank the Cyanide (CN) during the first 24 hours of management. He received cyanide incidentally, had lunch with the cyanide, went to the restroom where he became unconscious. First aid delivered by bystanders, with no Cardiopulmonary Resuscitation (CPR) required, as he had a weak pulse and was spontaneously ventilating. He remained unconscious for 10 minutes, prompting a call to the emergency services who transported and admitted to the local hospital. After 20 minutes, he noted to have no palpable central pulse and agonal breathing patterns with Pulseless Electrical Activity (PEA), thus prompting CPR as per Advanced Cardiac Life Support (ACLS) algorithm for 10-minutes after which Return Of Spontaneous Circulation (ROSC) occurred. A surface 12-lead electrocardiogram (ECG) showed J point and ST segment elevation of 4 mm in lead V2, and a saddleback ST segment elevation in lead V3 (Figure 1).
Investigations and management
He remained unconscious, was apyrexial and a head computed tomography performed, excluding intracranial pathology. Baseline laboratory tests revealed normal hemoglobin, white cell count, platelets and electrolytes. The patient was intubated in a spontaneous coma with minimal breathing effort thus requiring mechanical ventilation, blood pressure of 50/30 mmHg despite vasoconstrictor (noradrenalin) and inotrope (dobutamine).
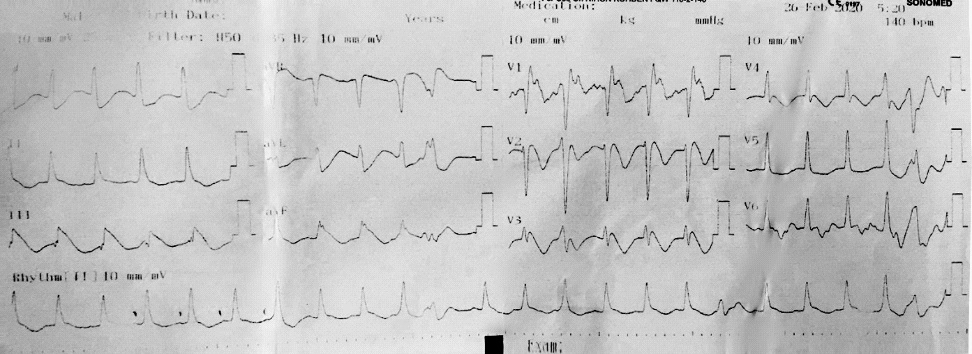
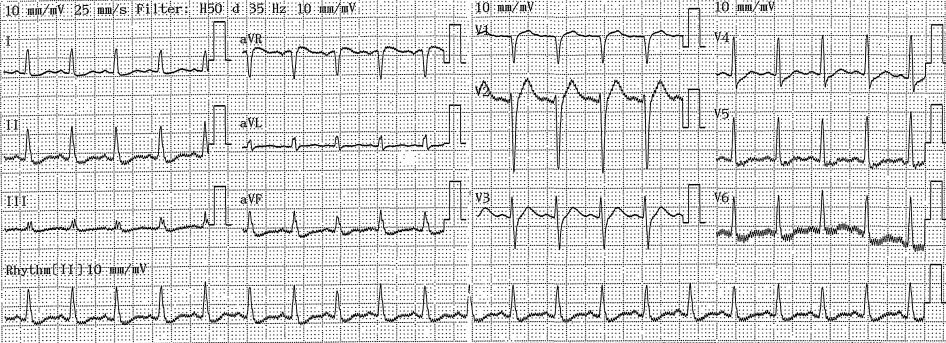
He was transferred to the intensive care unit, where he continued to have PEA, requiring resuscitation as per ACLS thus prompting VA-ECMO. The patient's arterial blood gas revealed severe metabolic acidosis with severe hyperlactatemia (pH 6.95; pCO2 16 mmHg; HCO3 - 3.5 mmol/l; BE -26,4 mmol/l; Lactate >15 mmol/l). Baseline laboratory tests performed on arrival to the hospital showed elevated serum TroponinT (77.3; normal ≤ 14 ng/l), normal white cell count and pro-calcitonin. The patient developed multi-organ failure requiring continuous renal replacement therapy (CRRT). The patient's ECG changed with T waves inversion noted and ST segment elevation of 5 mm in lead DIII, aVF (Figure 2) and a 12-lead ECG in sinus rhythm without ST elevation (Figure 3).
The toxic blood panel returned positive for cyanide poisoning. Then, investigation authorities discovered patients poisoning with much cyanide before 30 minutes of the onset of symptoms. After 32 hours, the patient died with a terminal rhythm of the asystole, despite initiation of antidotes.
A post-mortem examination showed the heart in the standard anatomical position of normal mass, dimensions and wall thickness. Opening the pericardium revealed pale red fluid <100 mls with some small hemorrhages on the surface of the left ventricle, all likely from prolonged CPR. The myocardium was soft with unobstructed epicardial coronary arteries (minor atheromatous plaques) with normal course and origin. The valves were normal morphology, no evidence of mitral valve prolapse, bicuspid aortic valve, or endocarditis. We confirmed the diagnosis of cyanide poisoning by the concentrations of cyanide in the blood, the stomach, and several organs.
Discussion
BrS is characterized by ST segment elevation in the right precordial leads (V1 to V3) of the ECG. The ST elevations in BrS may present intermittently, and these changes may be triggered or exaggerated by Na+ channel blockers (flecainide, procainamide or ajmaline) or other factors (high fever, alcohol or cocaine abuse). In the presence of weak Na+ current, theunopposed outward K+ current Ito and ICa, cause accentuation of the action potential notch in the RV epicardium. As these changes occur in the epicardium but not in the endocardium, they create a transmural voltage gradient resulting in accentuated J wave and ST segment elevation associated with the Brugadapattern [1].
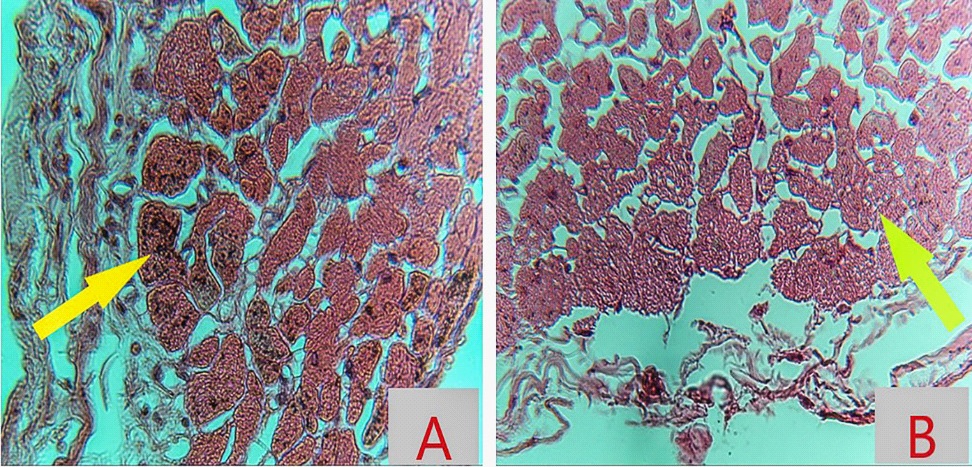
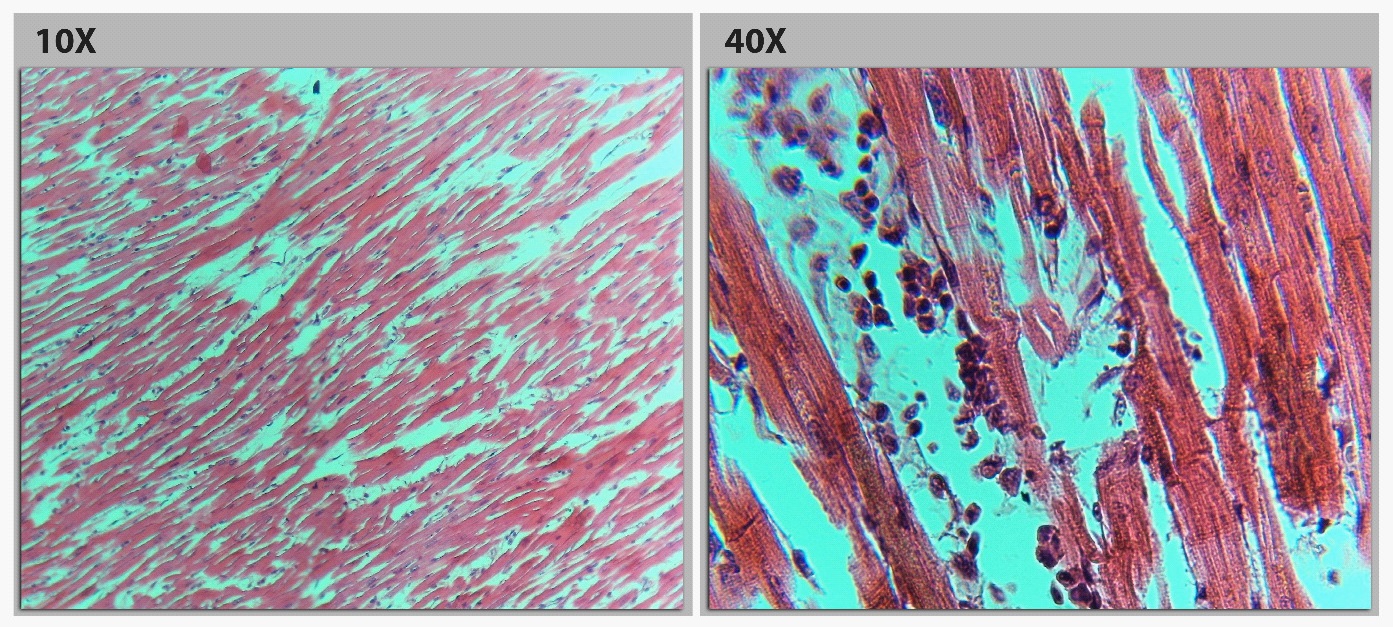
Our patient, a healthy middle-aged man, had no cardiovascular risk factors, no personal or familial history of syncope, sudden unexpected deaths or Brugada, annual ECG for 15 years has been normal, excluded the possibility of underlying Brugada syndrome, developed a type 1 Brugada pattern in the early hours of cyanide poisoning. The study on 161 patients with CN poisoning showed that there were five patients whose elevated ST segments and seven patients whose negative T waves [2]. The study of Zoltani et al, performance computational study of the cellular processes of ventriculur cardiac tissue exposed to CN is presented. The ECG pattern was generated from the computer resembling BrS calculated by computational algorithm following Luo-Rudy formalism, which relies on the change of sodium, potassium, and calcium currents directly by CN in the epicardium [3]. After exposure with CN, the concentration of CN is highest in the myocardium, its interference with the respiratory chain within the mitochondria of myocardial cells, especially in epicardium where the bloodstream reaches first and the number of Ito channel is more than other areas. As the consequence, the myocardial cells on the epicardium are the primary injury area. Bedsides, the repolarization phase of myocardium starts beginning from the epicardium to endocardium. So, the changes of ion channels in epicardium cells by CN effect in the early period create a transmural gradient between marginal (epicardium) and inner (endocardium) layers of the myocardium which results in ST changes and the appearance of a J wave that closely resembles the ECG in BrS. This mechanism may be suitable for the histopathological RVOT (Right Ventricular Outflow Tract) myocardiumfrom our patient. The RVOT epicardium region had the interstitial infiltration of fluid (oedematous liquid) and many eosinophils. Striations and nucleus within myocardial muscle cells are lost. The cytoplasms have many unknown black scattered throughout the entirety of cell (Figure 4A), which other areas such as endocardium and mid myocardium do not have. In some fields, the cytoplasms mimicked the alveoli of lung (Alveolarization), may be the next stage after degeneration of the unknown black (Figure 4B).
The endocardium and mid myocardium of RVOT were still striated and nucleus. The eosinophil infiltrated in the interstitial tissue (Figure 5). The absence of unknown black scattered in RVOT endocardium and mid myocardium excluded the mechanical damage due to chest compression.
The effects of CN on myocardial electrophysiology are not well known. It is difficult to determine clearly if all of the ECG changes were a direct consequence of CN poisoning or secondary to the severe metabolic disorder placed on the patient from CN toxicity. An early study carried by Wexler and colleagues showed direct changes to the myocardium in response to CN, which may explain some of the ECG changes. The authors demonstrated the electrical changes consisted of P-wave suppression and bradycardia, eventually culminating in ventricular tachycardia and Ventricular Fibrillation (VF) [1]. Patients with BrS are prone to VF and arrest which did not occur in our case.
The cause of the arrest with PEA was due to severe respiratory dysfunction at the cell. Because being absorbed into the body, CN moves through the cytoplasmic membrane and accumulates in the mitochondria hiding the respiratory chain. The authors suggest that restoring the depolarizing sodium current and maintaining calcium hemostasis are the most effective way to reverse these effects in CN poisoning. The CRRT is suitable to regulate any metabolic disturbances and as a method of thiocyanate clearance. Our patient also underwent hemodiafiltration. However, our patient was poisoned with a high dose and for quite a long time from exposed moment to diagnosis time, then finally he could not recover because of the severe cerebral tissue edema along with circulatory collapse.
Conclusions
CN toxicity can cause the ECG BrS pattern, particularly in the early hours. In this situation, the difficulty diagnoses early between the BrS and CN toxicity. The distinction is essential because CN toxicity early diagnosis and the antidotes treatments given immediately that the patient's prognosis will significantly improve. Therefore, it is necessary to have more research clarifying the mechanism of CN toxicity on the cardiovascular system and cardiac electrophysiology.
Learning objectives
1. CN toxicity can cause the type 1 BrS ECG pattern.
2. The histopathology of patients with CN toxicity has more
abnormalities in the epicardium than the endocardium.
3. Compare to BrS, the ECG of CN toxicity may be dynamic
and cardiac arrest in this case was PEA and asystole, not ventricular tachyarrhythmia.
References
- Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol. 2006; 29: 1130-1159.
- Jean-Luc Fortin, Thibault Desmettre, Cyril Manzon, et al. Cyanide poisoning and cardiac disorders: 161 cases. The Journal of Emergency Medicine. 2010; Vol 38, No 4: 467-476.
- Zoltani C, Platoff G, Baskin S. Acquired Brugada-like symptoms of cyanide caused cardiac toxicity: a computational study. Computers in Cardiology. 2004; 31: 281-284.
- Wexler J, Whittenberger JL, Dumke PR. The effect of cyanide on the electrocardiogram of man. American Heart Journal. 1947; 34: 163-173.