Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
Postpartum chest pain and the Hamman syndrome
Kieran VJ Mullins1*; Gideon Mlawa2
1 Registrar in Acute Internal Medicine, Department of Acute Internal Medicine, Queen’s Hospital, Rom Valley Way, Romford, London, RM7 0AG, UK.
2 Consultant Endocrinologist, Diabetelogist and General Physician, Department of Acute Internal Medicine, Queen’s Hospital, Rom Valley Way, Romford, London, RM7 0AG, UK.
*Corresponding Author: Kieran VJ Mullins
Registrar in Acute Internal Medicine, Department
of Acute Internal Medicine, Queen’s Hospital, Rom
Valley Way, Romford, London, RM7 0AG, UK.
Email: kieran.mullins@nhs.net
Received : Oct 30, 2021
Accepted : Dec 21, 2021
Published : Dec 28, 2021
Archived : www.jcimcr.org
Copyright : © Mullins KVJ (2021).
Abstract
A 17-year-old primigravida developed acute onset postpartum pleuritic chest pain following a prolonged second stage. The clinical syndrome rapidly progressed to chest, neck and facial swelling. Clinical evaluation in parallel with urgent investigations led to the diagnosis of Hamman syndrome, a rare though important benign cause of postpartum chest pain and shortness of breath. Conservative management consisting of close observation, supplemental oxygen, analgesia and reassurance lead to complete resolution of signs and symptoms by the third postpartum day.
Citation: Mullins KVJ, Mlawa G. Postpartum chest pain and the Hamman syndrome. J Clin Images Med Case Rep. 2021; 2(6): 1522.
Introduction
Hamman syndrome is a well-recognised though rare complication of pregnancy and related conditions where prolonged increases in intra-thoracic pressure may result in pneumomediastinum and subcutaneous emphysema. Clinicians should be aware of this benign clinical entity in order to facilitate a rational approach to investigation and management.
Case description
We present a case of a 17-year-old Caucasian primigravida who presented at 39 weeks gestation to the labour ward at our institution proceeding to deliver a healthy baby by normal vaginal delivery. In the late second stage of labour, the patient developed subcutaneous emphysema with pneumomediastinum. She had concomitant sudden onset of right facial and neck swelling and chest pain. On review of the birthing history she had a prolonged second stage of labour and had required episiotomy and vacuum extraction. She had no significant past medical history and had an uncomplicated antenatal course attending all scheduled appointments in the United Kingdom.
Examination of the patient revealed a young woman with normal BMI in mild respiratory distress speaking full sentences. Her vital signs were recorded: BP 136/80, HR 89 beats per minute, RR 22 breaths per minute, peripheral haemoglobin saturation via pulse oximeter 100% on ambient air and temperature 36.3 degrees Celsius. Examination of the chest wall was significant for diffuse bilateral palpable subcutaneous crepitation with extension of this sign to the neck and face. Chest auscultation was clear and heart sounds were preserved with no rubs, gallops or murmurs. The remainder of the examination was normal.
The differential diagnosis for this clinical presentation is broad and includes potentially catastrophic entities with high associated mortality including pneumothorax with or without tension, pulmonary embolism, amniotic fluid embolism, acute coronary syndrome, acute aortic syndrome, cardiac tamponade, and acute mediastinitis. Thus a structured and rational sequence of diagnostic investigations is paramount.
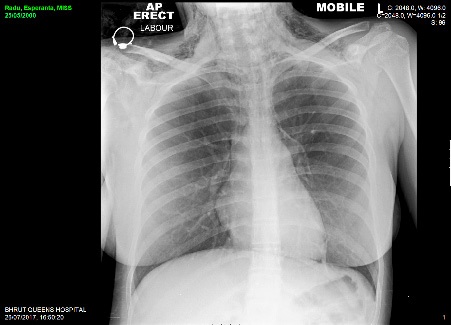
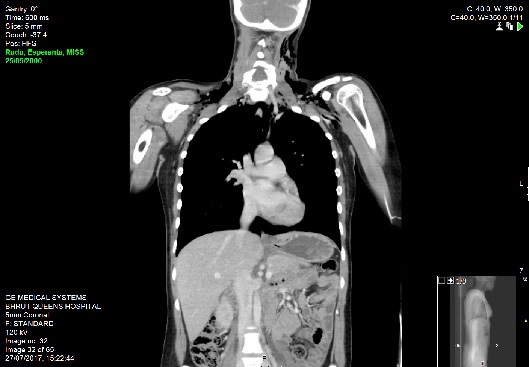
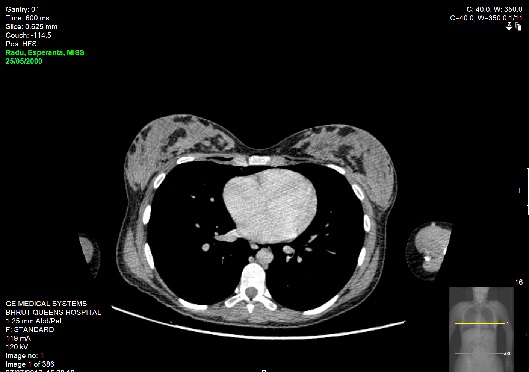
Emergent bedside electrocardiography demonstrated sinus tachycardia without axis deviation, evidence of ischaemia or right heart strain. Laboratory investigations revealed Hb 116, WCC 21, MCV 79 Sodium 134, potassium 3.8, ALP 341, Bilirubin 8, ALT 17, calcium 2.41. Arterial blood gas sampling was not performed as her SpO2 was 99% to 100% on ambient room air thoroughout her evaluation. Chest radiography demonstrated extensive subcutaneous emphysema and pneumomediastnum. Subsequent CT imaging of her thorax, abdomen and pelvis with intravenous contrast confirmed the finding of pneumomediastinum and extensive surgical emphysema spreading into the anterior chest wall and the head and neck. There was no evidence of pneumothorax or pulmonary embolism.
The patient was treated conservatively with close observation for 24 hours, supplemental oxygen and opiate analgesia in post-natal HDU. She was stepped down to the ward at 24 hours and discharged home at forty-eight hours by which time her symptoms had completely abated.
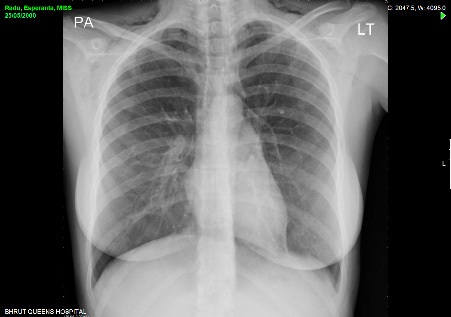
Pre-discharge repeat chest radiograph at 48 hours revealed improving subcutaneous emphysema. Unfortunately the patient did not re-present for follow up review at two weeks and was lost to long term follow up.
Discussion
Spontaneous pneumomediastinum is characterised by the presence of free air in the mediastinum. When it occurs in conjunction with subcutaneous emphysema it is termed the Hamman syndrome, first described by American physician Louis Hamman in 1945 in a postpartum female [1]. It is a rare clinical entity in pregnancy with an incidence of approximately 1 in every 100,000 deliveries [2,3]. A review of the literature reveals there have been around 200 reported cases over the last century; Hamman’s syndrome occurs more commonly in non-obstetric cases with 70.2% of those affected represented by young males in their early twenties [4].
The syndrome occurs without a traumatic antecedent and may occur without any identifiable precipitant. Classically, it is associated with a ‘crunching’ sound - Hamman’s sign - in synchronicity with the cardiac cycle, though such crunching is not universally present, nor is it necessary to make the diagnosis [5]. Though distressing Hamman’s syndrome is completely benign and self-limiting [6]. It is however, important to distinguish it from more serious clinical entities with similarly dramatic presentations such as cardiac tamponade, dissecting aortic aneurysm, mediastinitis, pulmonary embolus and the acute coronary syndrome.
Though it has been described during all stages of labour Hamman’s syndrome is most associated with a prolonged second stage [7]. Additional risk factors include primigravid women and cephalopelvic disproportion. It is thought occur following alveolar rupture secondary to increased intrathoracic pressures generated by maternal pushing, in effect repeated Valsalva maneuvers (forced expiration against a closed glottis). Pressures approaching 50 cm of water have been recorded resulting in the condition. The escaped air first traverses the perivascular space and mediastinum before spreading to involve the subcutaneous and retropharyngeal spaces of the neck. Ultimately the whole body may be involved [8]. Hamman’s syndrome is not limited to complicated labours and has been described following normal labour and following normal birthweight gestations [9]. Rarely, it has been reported in the antenatal period in the context of hyperemesis [10]. Other non-obstetric conditions which may cause subcutaneous emphysema with pneumomediastinum include status asthmaticus, oesophageal rupture or following trauma [11].
The clinical presentation depends upon the amount of air which has leaked into the free space. In the majority of cases subcutaneous emphysema and pneumomediastinum occur during labour or immediately postpartum. Symptoms may include acute pleuritic chest pain, dyspnoea and facial swelling. Subcutaneous crepitus is an inconsistent finding on clinical examination though when present in the correct clinical context is almost pathognomonic of the condition.
Hamman’s syndrome is a diagnosis of exclusion and the clinician must remain vigilant for alternative important and potentially life-threatening causes of chest pain and breathlessness such as pneumothorax, pulmonary embolism and acute coronary syndrome. These should be excluded with careful clinical correlation aided by judicious use of laboratory and radiological investigations. Clinicians should also include amniotic fluid embolism in the list of differentials. This is a rare cause of postpartum dyspnoea with no reliable clinical features and represents an obstetric catastrophe with very high mortality.
Hamman’s syndrome is self-limiting and with a good prognosis especially in mothers with no underlying cardiorespiratory compromise. However, emergent caesarian section may be indicated in some instances if subcutaneous emphysema and pneumomediastinum develop before birth, in order to avoid worsening or expansion of pneumomediastinum. Treatment is conservative with analgesia, oxygen and close monitoring. There is a need for close maternal monitoring during labour and postpartum in subsequent pregnancies.
Conclusion
Treating clinicians should suspect Hamman’s syndrome in any postpartum female with dyspnoea, chest pain and subcutaneous palpable crepitus particularly with prolonged second stage/difficult labours.
References
- Hamman L. Mediastinal Emphysema. J Am Med Assoc. 1945; p. 1-6.
- Arroyo M. Subcutaneous emphysema during labour. Int J Obstet Anesth. 2001; 10: 307-308.
- Karson EM, Saltzman D, Davis MR. Pneumomediastinum in pregnancy: Two case reports and a review of the literature, pathophysiology, and management. Obstet Gynecol. 1984; 64: 39S-43S.
- Brand M, Bizos B, Burnell L. A review of non-obstetric spontaneous pneumomediastinum and subcutaneous emphysema. South African Journal of Surgery. 2011; 49: 135-136.
- Hamman L. Spontaneous interstitial mediastinal emphysema of the lungs. Tr Asoc Am Physicians. 1937; 52: 311-319.
- Langwieler TE, et al. Spontaneous pneumomediastinum. Ann Thorac Surg. 2004; 78: 711-713.
- Duffy BL. Post partum pneumomediastinum. Anaesth Intensive Care. 2004; 32: 117-119.
- Nieboer B, et al. Pneumomediastinum as a cause of acute chest pain postpartum. J Matern Fetal Neonatal Med. 2006; 19: 243- 245.
- Reeder SR. Subcutaneous emphysema, pneumomediastinum, and pneumothorax in labor and delivery. Am J Obstet Gynecol. 1986; 154: 487-489.
- Seidl JJ, Brotzman GL. Pneumomediastinum and subcutaneous emphysema following vaginal delivery. Case report and review of the literature. J Fam Pract. 1994; 39: 178-180.
- Macklin M, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiment. Medicine. 1944; 23: 281-358.