Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 2
Effects of streptococcus mutans on the expression of inflammatory cytokine TNF-α and p53 by human gum fibroblast cells
Mohamadali Shadkam1; Noushin Jalayer Naderi2*; Ronak Bakhtiari3; Mahsa Oboodiat4
1 Faculty of Dentistry, Shahed University, Tehran, Iran.
2 Department of Oral and Maxillofacial Pathology, Faculty of Dentistry, Shahed University, Tehran, Iran.
3 Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
4 School of Dentistry International Campus, Tehran University of Medical Sciences, Tehran, Iran.
*Corresponding Author: Noushin Jalayer Naderi
Associate Professor, Department of Oral and
Maxillofacial Pathology, Faculty of Dentistry, Shahed
University, Tehran, Iran.
Email: Jalayer@shahed.ac.ir
Received : Oct 18, 2021
Accepted : Dec 22, 2021
Published : Dec 29, 2021
Archived : www.jcimcr.org
Copyright : © Naderi NJ (2021).
Abstract
Oral bacteria are involved in the etiology of oral cancer. In this study, Streptococcus mutans were examined for the gene’s expression involved in inflammation, carcinogenicity, and tumor inhibition among these species. This study aimed to determine the association between microbiome and oral cancer.
Human Gingival Fibroblast (HGF) and S. mutans were cultured on their relative mediums. Then, HGF was preconditioned whit several concentrations of S. mutans extract, and the cell viability was determined by MTT assay. HGF cells were treated with an optimum dose of bacterial extract, and then the total RNA was extracted from treated and untreated HGF cells. Lastly, p53 and TNF-α gene expression was evaluated by Real-Time PCR.
The results implied that in the S. mutans presence, TNF-α and P53 genes expression in the HGFs increased and decreased, respectively. This result suggests that S. mutans may invasively enhance oral cancer cells.
These findings more designate the association between oral microbiota and oral cancer. This relation is of clinical importance for the prevention and treatment of oral cancer in the future.
Keywords: streptococcus mutans; human gingival fibroblast (HGF); oral cancer; P53; TNF-α.
Citation: Shadkam M, Naderi NJ, Bakhtiari R, Oboodiat M. Effects of streptococcus mutans on the expression of inflammatory cytokine TNF-α and p53 by human gum fibroblast cells. J Clin Images Med Case Rep. 2021; 2(6): 1525.
Introduction
Streptococcus mutans species are the leading etiological factor of plaque formation and tooth decay [1]. In addition, these pathogens can display structural segments recognized by the host receptors. They can initiate inflammatory signaling pathways and intensifying cytokine production [2]. S. mutans has been shown to play a principal role in initiating caries, and it has also been the cause of lesions and gingivitis.
Periodontal diseases, such as gingivitis and periodontitis, are inflammatory infections. They are the most common oral health conditions that may eventually lead to severe chronic conditions in the oral cavity [3]. Inflammation appears to be associated with the growth of microorganisms, which is related to the production of inflammatory cytokines and linked to the destruction of oral tissues and the release of harmful nutrients. These events can create an inflammatory microenvironment and produce mediators of oxidative stress by forming periodontal pockets and gingival tissue, alveolar bone, and periodontal ligament destruction [4]. Inflammation involves immune cells and several mechanisms that act at different levels, including changes in immune cells arrangements in tissues, changes in the cell’s response to inflammatory stimuli, regulation of signaling pathways, and control of gene expression [5]. Inflammation can be a cause of cancer. Bacteria can stimulate the cell by activating the NF-κB pathway and inhibiting the cell apoptosis, then spread several types of cancer
The p53, a tumor suppressor gene, is a cancerous gene inactivated by mutations that lead to the loss of function. Inactivation of these genes eliminates the tissue homeostasis that characterizes an expanding tumor. TNF-α stimulates matrix metalloproteinase and prostaglandin production by fibroblasts. TNF-α is a crucial mediator of the inflammatory response [6].
Human Gingival (or Gum) Fibroblasts (HGF) are the most abundant cells living in the oral mucosa. Paracrine factors they produce may play a key role in gingivitis and periodontal tissue rehabilitation. HGF cells support the tooth structures, as they are the rich source of MSCs [4].
Accordingly, this study aimed to investigate the role of Streptococcus mutans, a most important cariogenic bacterial species, and its metabolites in the cancer induction in the healthy gingival cell line by measuring the expression of P53 and TNF-α genes in HGF cells.
Material and methods
Streptococcus mutans procurement and culture
We selected Streptococcus mutans (ATCC-25175) as an oral pathogen for the study, purchased from the Iranian Biological Resource Center. S. mutans was cultured to a Brain Heart Infusion broth (BHI, Difco Laboratories) medium plus streptomycin, and incubated overnight at 37°C [7].
Genotypic characterization of the S. mutans
Meanwhile, the biochemical identification methods for some bacteria have had not been truthful, amplification and sequencing of the 16S rRNA performed to confirm bacterial credentials. However, S. mutans was obtained from an international valid biobank, we just confined the PCR method for its credential.
DNA extraction
Genomic DNA was extracted from bacterial mass using the Bacterial Genomic DNA Isolation Kit (NORGEN BIOTEK, Cat. 17900, Canada) as per manufacturer’s instructions [8]. Briefly, DNA extraction performed in 4 steps;
1. Lysate Preparation: Bacterial culture (Gram-Positive Bacteria) was pelleted, and then, Solution A, lysozyme solution (400
mg/mL), and the Lysis Buffer P and Proteinase K were added in
order.
2. Binding to Column: The solution BX and absolute ethanol
added to the lysate, the resulting suspension poured into a spin
column.
3. Washing Bound DNA: The spin column was washed twice
by Wash Solution A.
4. Elution of Clean DNA was performed by Elution Buffer B.
Consequently, eluted DNA was analyzed on an 1% agarose gel
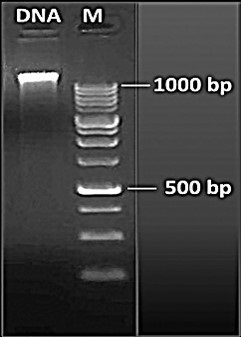
electrophoresis, adjacent to a 1-Kb DNA ladder.
Primers designing
The primers for S. mutans strain ATCC 25175 (NCTC 10449) 16S ribosomal RNA sequence (Gen Bank accession number AJ243965.1) were designed using the Primer-BLAST online tool. Randomly an oligonucleotide consists of almost 20 nucleotides were selected on the plus strand as a potential forward primer. Then another oligonucleotide was randomly chosen on the plus strand, but converting it to reverse and complement as if it is on the minus strand. Then, adding or omitting nucleotides tried to match the annealing temperatures, recorded in Table 1.
Table 1: Primer sequences of 16S ribosomal RNA gene for S. mutans, strain ATCC 25175 (NCTC 10449).
Primer |
Sequence (5'->3') |
Template strand |
Length |
Start |
Stop |
Tm(°C) |
GC% |
Product length |
Forward |
GCGGGGGATAACTATTGGA |
Plus |
19 |
122 |
140 |
56.26 |
52.63 |
413bp |
Reverse |
CGGGACCTACGTATTACCG |
Minus |
19 |
534 |
516 |
56.85 |
57.89 |
Table 2: Preliminary results include raw data.
Gene |
Type |
Reaction Efficiency |
Expression |
Std. Error |
95% C.I. |
P(H1) |
Result |
P53 |
TRG |
0.96 |
0.194 |
0.149 – 0.279 |
0.142 – 0.309 |
0.050 |
- |
TNF-α |
TRG |
0.93 |
11.556 |
8.487 – 15.537 |
8.186 – 19.766 |
0.000 |
UP |
B ACTIN |
REF |
0.91 |
1.000 |
- |
- |
- |
- |
PCR amplification
PCR for 16S rRNA gene was performed using primer. Each 25 µl volume of a PCR reaction mixture comprised 12.5 µl of 2 X buffer, dNTPs 200 µM, primers 1 µM, bacterial genomic DNA 1 µg and 2 unit of Taq PCR Master Mix Kit (QIAGEN, cat. no. 201445, US) [8]. The PCR amplification for specific 16SrRNA was performed using a thermal cycler (Bioneer, Germany).
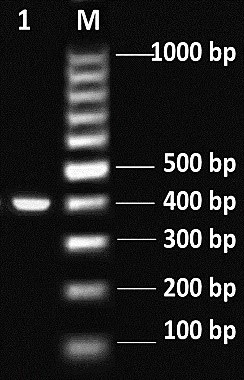
The ‘touchdown’ PCR conditions were as follows: Initial denaturation at 95°C for 15 min, continued by 35 cycles, denaturation at 94°C for 1 min, annealing at 60 °C for 1 min, extension at 72°C for 2 min. The program sustained by a final extension at 72°C for 10 min [8]. PCR products were assessed on 1% agarose gel electrophoresis, besides a100-bp DNA ladder (Fermentas, Germany).
PCR product purification
This product was purified using the Pure Link™ Quick Gel Extraction Kit (Thermo Fisher Scientific, kit no. K210012, US), following supplier’s instructions. In brief, to clean up PCR product from the agarose gel, two 50 µl PCR reaction performed as mentioned earlier. Then, total both products were run on a 2% agarose gel. The kit procedure lays on first gel solubilization, binding the target to the provided columns, then washing and eluting followed by DNA purification.
16S rRNA fragment sequencing
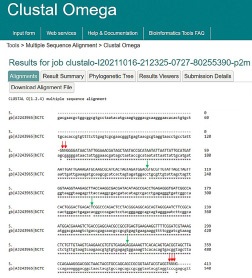
Purified DNA was sent for sequencing at a commercial service provider (Macrogen, Seoul, Korea) by Pyrosequencing technology. Using alignment in Clustal Omega online tool ofEMBLEBI (available at https://www.ebi.ac.uk/Tools/msa/clustalo/#), our given sequence was aligned with the sequence of 16S ribosomal RNA from S. mutansstrain (Gen Bank accession number AJ243965.1). Then, NCBI BLASTn (available at http://blast.ncbi. nlm.nih.gov) was employed to construct phylogenetic trees from evolutionary distance data in neighbor-joining method.
Bacterial conditioned medium (CM) preparation
The cultured bacteria were centrifuged at 4000 g for 15 min to separate the biomass and cultural liquid. Then, the supernatant cleared through a 0.45 μm sterile syringe filter(Millipore filter Co., Bedford, MA). The obtained conditioned medium contained secretion factors produced by S. mutans during the proliferation.The clarified medium was then concentrated 25-fold using a 10 kD pour size amiconcentricon centrifugal concentrator (Millipore Corp., Bedford, MA)[9].
HGF cell line providing and culture
The Human Gingival Fibroblast (HGF) cell line (IBRC C10459) was acquired from the cell bank of Iran Stem Cell Technology Company. HGF cells were cultured,a density of 1.5 X 105 cells/ mL, in T25 culture dishes with 4 mL of complete medium, Dulbecco’s Modified Eagle’s Medium (DMEM High Glucose, Gibco, CA, USA). A completed medium consists of 90% DMEM supplemented with 10% fetal bovine serum (FBS, Gibco, CA, USA) and 1% Penicillin and Streptomycin antibiotics (Gibco, CA, USA).
HGF cell viability after exposer to S. mutans CM
The cell cytotoxicity of the S. mutans conditioned medium was evaluated against HGF cells. Primarily, we prepared a serial dilution of S. mutans CM. Formerly, 105 fibroblasts per 100 µL of the complete cell culture medium were seeded into each well of a 96-well plate and incubated overnight to let cells adhere. Then, cells were treated with each concentration (1-, 0.5-, 0.25-fold dilution) in three wells, and three wells of untreated cells were left as negative control. After 24 h of incubation, cell viability was assessed using10 µL MTT (5 mg/mL in PBS, Sigma, USA) solution for each well [10], as first described by Mosmann [11] with the proposed modifications by Denizot [12].
HGF cell line treatment with CM
Following HGF cell growth, they were passaged but now without supplement to assess the CM influence alone. Simultaneously, 1µl/ml of 25-fold CM was added to the HGF cell culture, and then cells were harvested after overnight incubation [9].
Assessment of cancer induction in HGF cells after exposure to S. mutans’s CM
Total RNA extraction: Total RNA was extracted from CM-HGF and HGF using the RNX plus Kit (Cinnagen, Iran), following the instruction. Briefly, HGF cells were harvested after 24 hours pretreatment with S. mutans’s CM, by centrifugation. The supernatant was removed, and cells were washed using sterile PBS. It was added 600 μl of RNX solution to lyse the cells, and the Lysate solution was centrifuged at 1500 rpm for 5 minutes. Then 200 μl of chloroform was added to the tube on ice or at 4°C for 5 minutes. In the end, total RNA concentration was identified with Nano-drop (Thermo Scientific ND-2000), and RNA integrity was assessed over 1% agarose gel electrophoresis.
cDNA synthesis: The cDNA was made from RNA extracted from HGF cells, using the Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher, K1622), according to the kit’s instructions. Briefly, 8 μl RNA, 1 μl of random hexamer primer, and depswater up to 12 μl were incubated in a microtube at 70°C for 5 minutes, and immediately placed in ice. Next, the 20 μL-final volume included 4 μl of 5X reaction buffer and 2 μl of dNTP mixture and incubated at 25°C for 5 minutes. After adding 1 μl Reverse Transcriptase enzyme, the microtube held on in a thermal cycler (Bioneer® , Korea), at 25°C for 10 minutes, 42°C for 60 minutes, and 70°C for 10 minutes.
Evaluation of P53 and TNF-α genes expression by real-time PCR: In this study, SYBER green method and β-actin gene (a housekeeping gene as an internal reference) was used to evaluate the P53 and TNF-α genes expression in fibroblasts treated with S. mutans. Briefly, for PCR reaction, 2 μl of cDNAs from treated and untreated HGF cells, 10 µl Master mix, 5 µl DEPC water, and then 1 µl of each primer (of P53, TNF-α, and β-actin genes) were added to each vial by the total volume of 20 µl. The reactions were performed in two steps, step 1: the initiation denaturation at 95°C for 10 minutes. The step 2 was 40 cycles consisted of denaturation at 95°C for 15 seconds, and annealingat 60°C for 60 seconds.
Statistical analysis
Real-time PCR data were analyzed using REST software to evaluate gene expression. The results were analyzed by nonparametric Mann-Whitney and ANOVA methods by SPSS software. Results were reported as mean standard deviation. Standards deviation analysis was used to evaluate the significance of group differences. P-Value Less than 0.05 was considered as significant results. All tests were performed in triplicate (repeated 3 times).
Results
S. mutans DNA Extraction
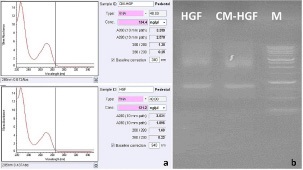
After overnight culturing of the standard bacteria and reaching the required turbidity, genomic DNA was extracted from. S. mutans. DNA, passing the extraction procedure, was analyzed on an 1% agarose gel electrophoresis, adjacent to a 1-Kb DNA ladder illustrated in Figure 1.
S. mutans genotypic characterization: The amplified 413-bp PCR product was runed on an 1% agarose gel electrophoresis, adjacent to a 100-bp DNA ladder showed in Figure 2.
16S rRNA fragment sequencing: Purified DNA, subsequent a clean-up procedure, sequenced in both directions. Deciphering the partial sequence of the 16S rRNA from S. mutans was performed using pyrosequencing technology. In sequencing pathway, as illustrated in Figure 3, there were 3 mismatches and the reading didn’t complete for both ends of the fragment. All these data confirmed that our microorganism is S. mutans.
HGF cell viability after exposer to S. mutans CM
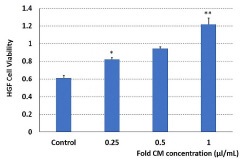
The cell-free conditioned medium from S. mutans culture supernatant was stored at −20°C. Following treatment of HGF cells with each dilution (1-, 0.5-, 0.25-fold dilution) of CM of S. mutans, it seems the higher the concentration of CM, the higher the growth and survival of cells. As depicted in Figure 4, cell treatment with 1 µl/mL not diluted CM (25-folded concentration, shown as 1 in the graph) and 0.5-fold dilution increased their viability significantly at the p<0.01and p<0.02, respectively. This result confirmed, S. mutans CM not only was non-toxic for the HGF cells but also stimulated the cell proliferation.
Cell culture and pretreatment of HGF
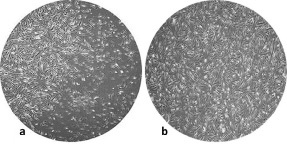
Cell culture was observed daily by 10X magnification of the contrast phase microscope. Based on these observations, clones of fibroblastic cells appeared in the initial culture. These cells retained their fibroblast morphology during culture. The growth rate of the cells increased after treatment, so these cells filled the plate surface more within three days. Figure 5 displays HGF microscopic image at 90% confluency and HGF image when exposed by S. mutans CM.
Assessment of cancer induction in HGF cells after exposure to S. mutans CM
Total RNA extraction: Quantitative evaluation of extracted RNA was performed using a nanodrop for the concentration assessment (Figure 6a). And qualitative evaluation of the isolated RNA was performed using 1% agarose gel electrophoresis. RNAs concentration illustrated by nanodrop were 134.4 and 121.2 ng/μl for CM-treated and non-treated HGF cells, respectively (Figure 6b).
Discussion
S. mutans, as Lactic Acid Bacteria (LAB), provides its energy supply only from glycolysis [13]. It is a principal etiological agent of human dental caries (cariogenic), a frequent component of dental plaque, and live independently in biofilms. S. mutans various high-affinity surface adhesins allow colonization [14].
Several studies pointed out the physiological role of S. mutans in the cytotoxic reduction of HGF cells and not producing toxic effects themselves [13,15-17]. Agree with them, our in vitro results, evaluated CM treated HGF cells' survival by MTT assay after overnight incubation, showed an increased cell growth. Cell viability improved in a dose dependent manner. Even though, the components released from S. mutans may be cytostatic for HGF cells rather than cytotoxic. The results obtained in our study showed that the significant increased HGF cell proliferation occurred at 0.5, and 1 µl/mL CM concentrations in the value of p<0.02 and p<0.01, respectively. While there is a lack of evidence for cytotoxic effects of S. mutans CM on HGF cells in the literature, we could not compare the results. Simply Kim, D. et al. in 2017 reported that sucrose breaks down to fructose and glucose forms in S. mutans CM [18]. So, probably the high concentration of fructose and glucose in CM can provide a sugar-rich micro-environment for HGF cells to grow more.
Although, regular exposure to S. mutans and its metabolites may demand the continuous renewal of HGF, the potential risk of cells conducted to oral cancer should not neglect. Consequently, we provoked to evaluate the expression of genes involved in cell carcinogenesis.
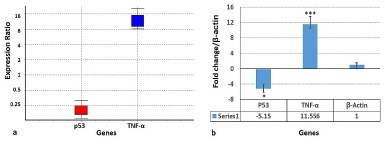
As the results of the real-time PCR test revealed, the tumor suppressor gene (p53) expression was decreased five times in HGF cells after overnight exposure to CM of S. mutans. The significant (p<0.05) reduction of P53 production inhibited cell apoptosis, leading to excessive proliferation of cells resulting in carcinogenesis. The inhibitory effect of bacterial soluble metabolites on the expression of p53 in HGF cells monolayers might be mediated by soluble factors properties or by adhesion molecules attributes.
The involvement of bacterial CM in the stimulation of the pro-inflammatory cytokine TNF-α expression in HGF gingival fibroblasts was investigated by the pretreatment of fibroblasts. Then, TNF-α expression showed eleven times increase in S. mutans CM treated HGF cells. The excessive production of TNF-α increases inflammation, which is itself a trigger of neoplasm.
However, it has been proven in the scientific data that TNF-α operates as a director switch in creating a complex association between inflammation and cancer [19]. TNF-α stimulates Matrix Metalloproteinases (MMPs) expression in HGFs at inflammatory sites in the gingival tissue [20,21]. MMPs are the principal mediators which destroy the connective tissue in periodontitis [20]. Accordingly, many MMP genes associate to cancer [22]. TNFα is a dominant cytokine and the intermediate of innate immunity and inflammatory responses. It is a potent stimulator of MAPks (ERK, JNK, and p38 MAP kinases). And MAPks, in turn, up-regulate TNFα expression [23]. The mechanism of the MAPKs signal pathway stimulates cell proliferation and may increase the risk of tumor formation. Hence, we can conclude that pro-inflammatory cytokines such as TNF-α are indirectly implicated in carcinogenesis induction.
To the best of our knowledge, this is the first report of the S. mutans CM’s effect on conducting HGF cells to malignancy. The study's objective was to investigate whether there are any associations between the S. mutans CM and HGFs go to malignancy?
Despite satisfactory results, there were some limitations in our study. One limitation was that the in vitro experiments are not sufficient to provide accurate data, and in vivo trials are required. Further, it is impossible to adequately speculate the outcomes of a complicated system including fibroblasts, multilayered epithelial cells, and defense cells because the study implemented in monolayer cells. These experiments, at least, should be conducted on the organoid-based models to consider the genetic structure and various individual responses. Another limitation was that obtained HGF cells from a single clone could not be generalized to the distinct age assortments and diverse genetic traits.
Conclusion
In conclusion, the results obtained in our study revealed that aberrant proliferation of HGF under the S. mutans CM exposure leads to down-regulating the p53 gene and up-regulating the TNF-α gene. Low expression of P53 and excessive production of TNF-α directs cells to become cancerous.
Declarations
Acknowledgements: Authors thank Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences for their kind help and cooperation throughout this research project.
Authors’ contribution: Shadkam, Naderi and Bakhtiari reviewed literature, outlined, wrote the manuscript. Oboodiat edited the manuscript and prepared figures. All authors read and approved the final manuscript.
Conflicts of interest: The authors declare that they have no conflicts of interest.
References
- Palmer Jr, R.J., Composition and development of oral bacterial communities. Periodontology 2000, 2014. 64: p. 20-39.
- Esteban-Fernandez, A., et al., The role of wine and food polyphenols in oral health. Trends in Food Science & Technology. 2017; 69: p. 118-130.
- Pihlstrom B.L., B.S. Michalowicz, and N.W. Johnson, Periodontal diseases Lancet 366: 1809–1820.
- Costantini E., et al., Assessment of the Vanillin Anti-Inflammatory and Regenerative Potentials in Inflamed Primary Human Gingival Fibroblast. Mediators Inflamm. 2021. 2021: p.5562340.
- Chen L., et al., Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018; 9: p.7204-7218.
- Birkedal-Hansen H., Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993; 28: p. 500- 510.
- Nomura R., et al., Contribution of Streptococcus mutans to Helicobacter pylori colonisation in oral cavity and gastric tissue. Sci Rep. 2020; 10: p. 12540.
- Matsuyama J., et al. PCR for detection of mutans streptococci in human dental plaque. In International Congress Series. 2005. Elsevier.
- Saeedi, P., R. Halabian, and A.A.I. Fooladi, Mesenchymal stem cells preconditioned by staphylococcal enterotoxin B enhance survival and bacterial clearance in murine sepsis model. Cytotherapy. 2019; 21: p. 41-53.
- Khodavirdipour A., et al., To Study the Anti-cancer Effects of Shigella Flexneri in AspC-1 Pancreatic Cancer Cell Line in Approach to Bax and bcl-2 Genes. J Gastrointest Cancer. 2021; 52: p. 593- 599.
- Mosmann T., Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65: p. 55-63.
- Denizot F. and R. Lang, Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986; 89: p. 271-277.
- Lemos J.A., et al. The Biology of Streptococcus mutans. Microbiol Spectr, 2019; 7: p. 10.1128/microbiolspec.GPP3-0051-2018.
- Tang X., et al., Cariogenic Streptococcus mutans Produces Tetramic Acid Strain-Specific Antibiotics That Impair Commensal Colonization. ACS Infect Dis. 2020; 6: p. 563-571.
- Kajfasz J.K., et al., Transcriptome responses of Streptococcus mutans to peroxide stress: Identification of novel antioxidant pathways regulated by Spx. Sci Rep. 2017; 7: p. 16018.
- Peeridogaheh H., et al., The impact of Aggregatibacter actinomycetemcomitans biofilm-derived effectors following antimicrobial photodynamic therapy on cytokine production in human gingival fibroblasts. Photodiagnosis Photodyn Ther. 2019; 27: p. 1-6.
- Grande R., et al., NF-kB mediated down-regulation of collagen synthesis upon HEMA (2-hydroxyethyl methacrylate) treatment of primary human gingival fibroblast/Streptococcus mutans cocultured cells. Clinical oral investigations. 2015; 19: p. 841-849.
- Kim D., et al., Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep. 2017; 7: p. 41332.
- Wu Y. and B.P. Zhou, TNF-alpha/NF-kappa B/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010; 102: p. 639-644.
- Birkedal-Hansen, H., et al., Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993; 4: p. 197-250.
- Giannobile W.V., K.F. Al-Shammari, and D.P. Sarment, Matrix molecules and growth factors as indicators of periodontal disease activity. Periodontol. 2000, 2003; 31: p. 125-34.
- Itoh, Y. and H. Nagase, Matrix metalloproteinases in cancer. Essays Biochem. 2002; 38: p. 21-36.
- Sabio, G. and R.J. Davis, TNF and MAP kinase signalling pathways. Semin Immunol. 2014. 26: p. 237-245.