Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 3
Metastatic pancreatic ductal adenocarcinoma presented as a cervical mass and rectal mass: A case report
Po-man TSANG1; Chun-hai LO1; Cheuk-nam LING1; Hoi-lam CHEUNG2; Cheuk-lam HO3
1 Department of Pathology, United Christian Hospital, Hong Kong.
2 Department of Pathology, Tseung Kwan O Hospital, Hong Kong.
3 Department of Pathology, United Christian Hospital, Hong Kong.
*Corresponding Author: Lo Chun-Hai
Department of Pathology, United Christian Hospital,
Hong Kong.
Email: lch333@ha.org.hk
Received : Jan 14, 2022
Accepted : Feb 09, 2022
Published : Feb 16, 2022
Archived : www.jcimcr.org
Copyright : © Chun-Hai L (2022).
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of malignant tumour in the pancreas. It is a highly aggressive tumour with poor prognosis. The liver and peritoneum are the most common sites of metastasis, and metastasis to colon and cervix are extremely rare. We herein report a case of metastatic PDAC presented as a cervical mass and rectal mass. Biopsies were taken from the rectal mass, cervical mass, and pancreatic mass. They show similar morphology and same immunostaining results, including loss of nuclear staining for SMAD4. In viewing unusual morphology and a lack of precursor lesions in small biopsies, one should alert the possibility of metastasis. Correlation with clinical history and radiological examination and the use of appropriate immunohistochemistry are also essential for making the correct diagnosis.
Citation: Po-man T, Chun-hai L, Cheuk-nam L, Hoi-lam C, Cheuk-lam H. Metastatic pancreatic ductal adenocarcinoma presented as a cervical mass and rectal mass: A case report. J Clin Images Med Case Rep. 2022; 3(2): 1664.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of malignant tumour in the pancreas. It is a highly aggressive tumour with poor prognosis, and is one of the leading cause of cancer–related mortality worldwide [1,2]. The average 5-year survival rate is less than 10% [3], and the 1-year survival is less than 20% of for patients with metastatic disease [4]. Usually, patients present with weight loss, abdominal pain, and jaundice [5]. The liver and peritoneum are the most common sites of distant metastasis from PDAC, and other less common sites of metastasis are the lung, brain, kidney, and bone [6-8]. Metastasis to colon and cervix are extremely rare, with only a few case reports available in English literature [9- 15]. We herein report a case of metastatic PDAC presented as a cervical mass and rectal mass.
Case presentation
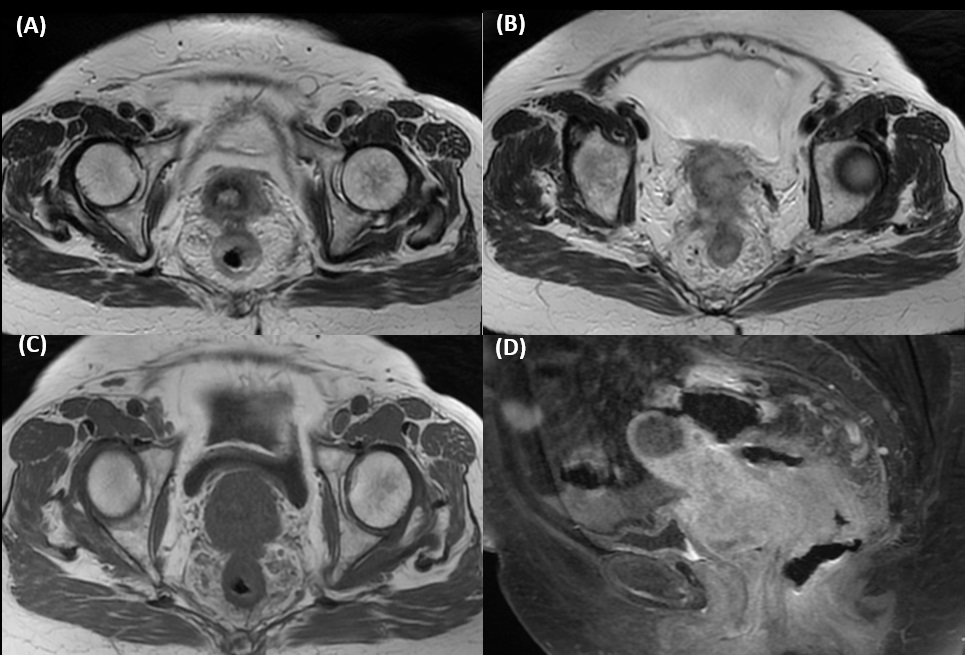
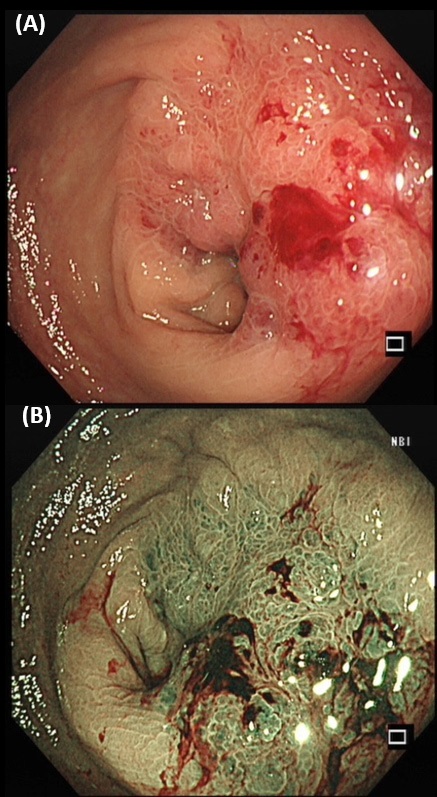
A 75-year-old lady, with history of hypertension, hyperlipidaemia and depression, was presented with on-andoff lower abdominal pain, occasional mild per-rectal bleeding (small amount of fresh blood stained on tissue paper) and loss of appetite for around 1 month. Physical examination showed no jaundice nor pallor. No abdominal mass nor enlarged lymph nodes were palpated. However, a circumferential mass was noted 5 cm from anal verge during per-rectal examination. Blood tests showed Hb level of 12.2 g/dL (reference range 11.5-15.4 g/ dL), total bilirubin 14 umol/L (reference range <22 umol/L), ALP 85 IU/L (reference range 35-104 IU/L), ALT 12 IU/L (reference range 10-35 IU/L), and creatinine 51 umol/L (reference range 44-80 umol/L). CEA level was 6.3 ug/L (reference range <4.7 ug/L). Magnetic Resonance Imaging (MRI) of the pelvis and colonoscopy were performed in view of the rectal mass. MRI of the pelvis showed near circumferential wall thickening in the rectum, which measured 7.7 cm in length and 1.7 cm in maximum thickness. Anterior and contiguous to this tumour was a large heterogenous enhancing lesion measuring 5.9 cm X 5.3 cm X 4.8 cm in size in the lower uterine body and uterine cervix, resulting in hydrometra (Figure 1). In colonoscopy, a nearcircumferential constricting tumor was noted 6 cm from anal verge (Figure 2). The rest of the colon could not be examined as the scope failed to pass through the tumour. Multiple biopsies were taken. Gynecologist was also consulted. Speculum and pervaginal exam showed cervix with smooth and normal mucosal surface. However, it is hardened with a few submucosal firm nodules. Transvaginal and transabdominal ultrasound showed gross ascites and fluid-filled endometrial cavity. There was no definite cervical mass or adnexal mass. Endometrial aspiration and biopsy from cervix were performed.
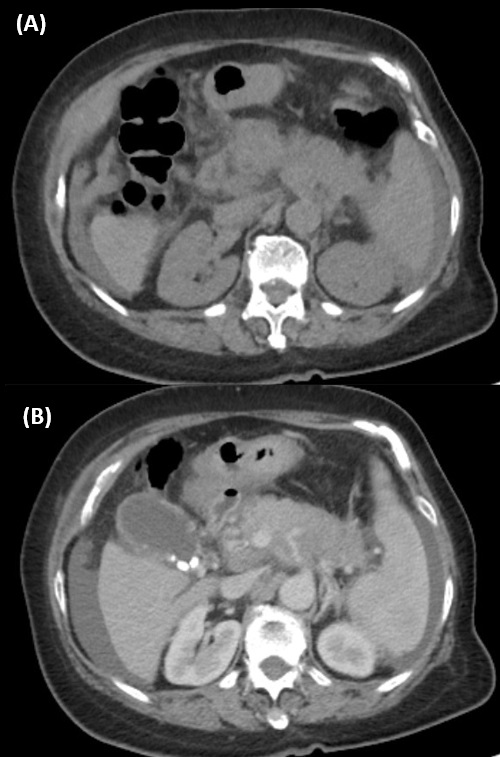
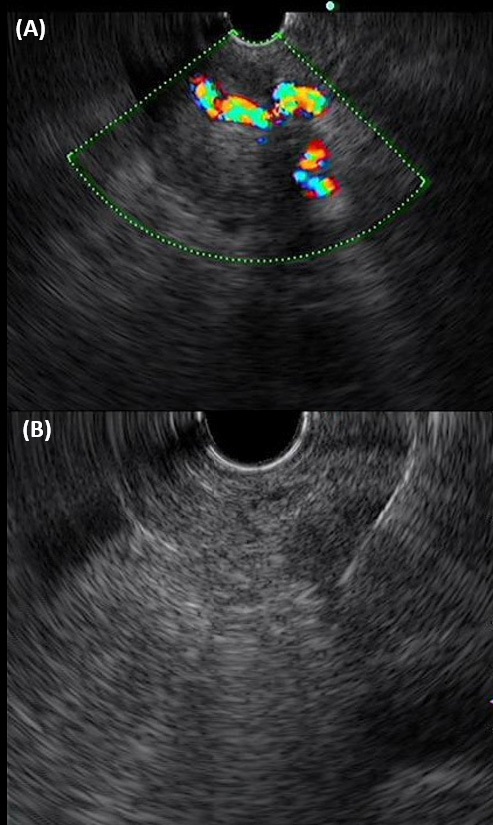
Later on, a PET-CT scan was also done, which showed the same near circumferential wall thickening in the rectum and hypermetabolic mass lesion measuring in the lower uterine body and the cervix (SUV max 8.1) as revealed by the MRI scan. Additionally, there was a hypermetabolic mass lesion measuring 2.6 cm X 2.8 cm X 2.9 cm in size in the left upper quadrant of the abdomen abutting onto the surface of the splenic flexure (SUVmax 9.1), suspicious for peritoneal metastasis. The body of pancreas is diffusely swollen, with ill-defined increase in FDG uptake (SUVmax 6.4) and encasement of the celiac trunk and its major branches, the splenic and portal vein (Figure 3). Features were suspicious for pancreatic carcinoma. A hypermetabolic aortocaval lymph node measuring 1 cm in diameter was noted (SUVmax 6.4), suspicious for nodal metastasis. No other suspicious hypermetabolic lesion was seen elsewhere in the body. The overall impression at that juncture was rectal cancer with uterine invasion, or uterine cancer with rectal invasion, and a suspected concurrent tumour in the pancreas. In view of the suspected concurrent pancreatic tumour found in PETCT scan, endoscopic ultrasound (EUS) of the biliary system and pancreas was also performed. A heterogeneous hypoechoic mass at pancreatic body and tail with encasement of splenic vessels was identified (Figure 4). Multiple biopsies were taken.
Microscopic examination
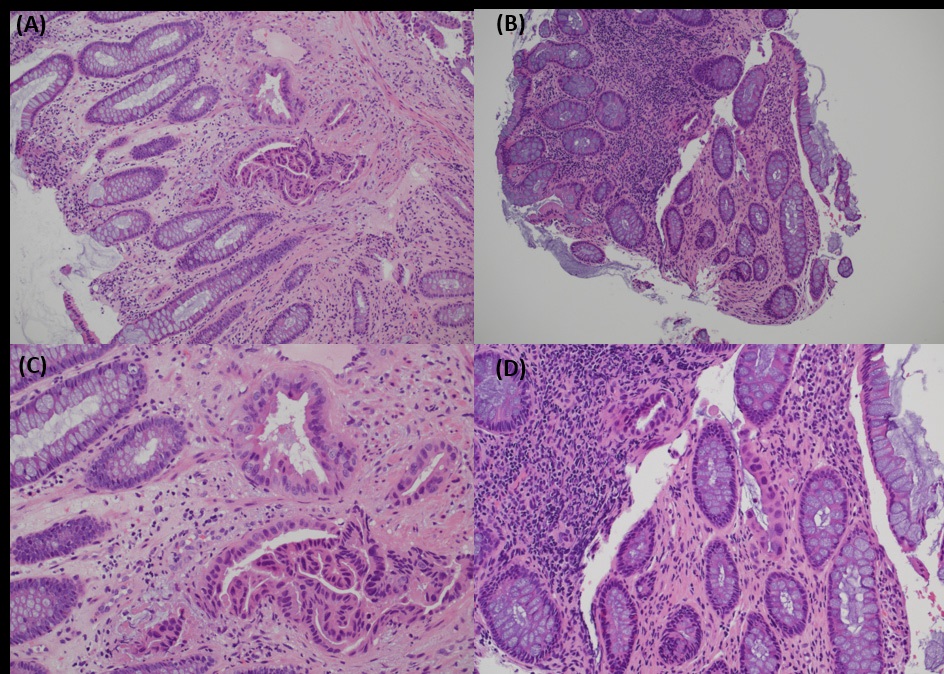
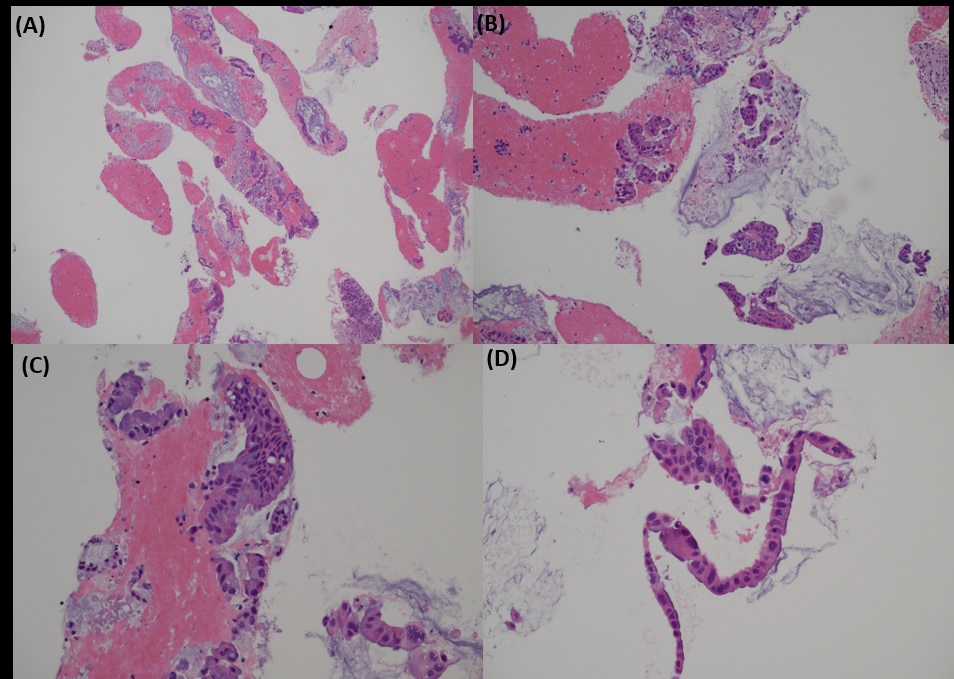
Biopsies taken during colonoscopy showed multiple pieces of colonic mucosae. There were two small foci of suspicious glands in the lamina propria. The glands were lined by cuboidal to low columnar cells with enlarged, hyperchromatic nuclei. The surrounding colonic glands showed no dysplasia. In view of the scarcity of suspicious glands, a definitive diagnosis was not possible. The report was signed out as suspicious of malignancy, and metastatic tumour had to be considered (Figure 5).
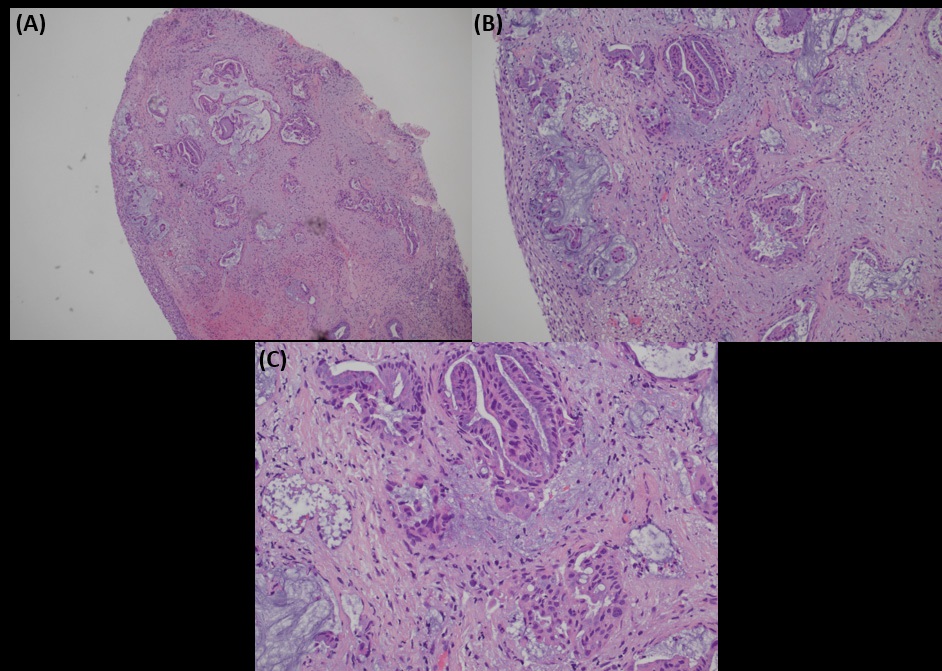
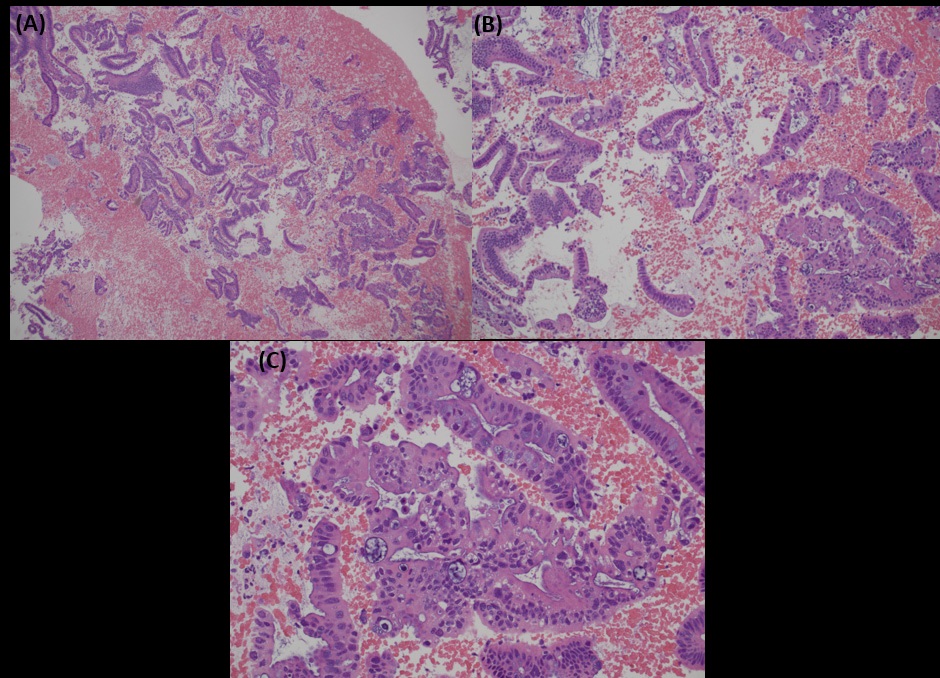
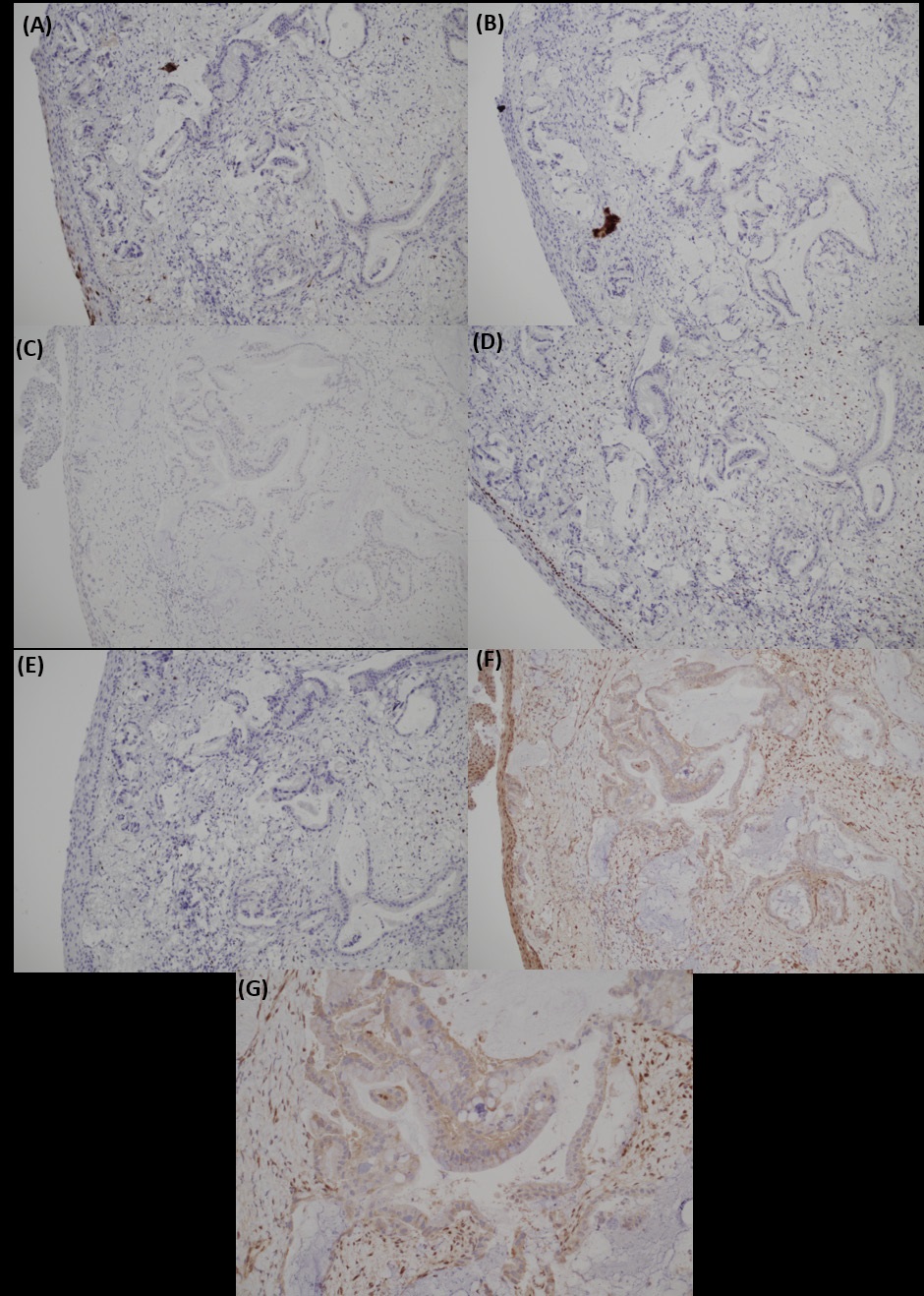
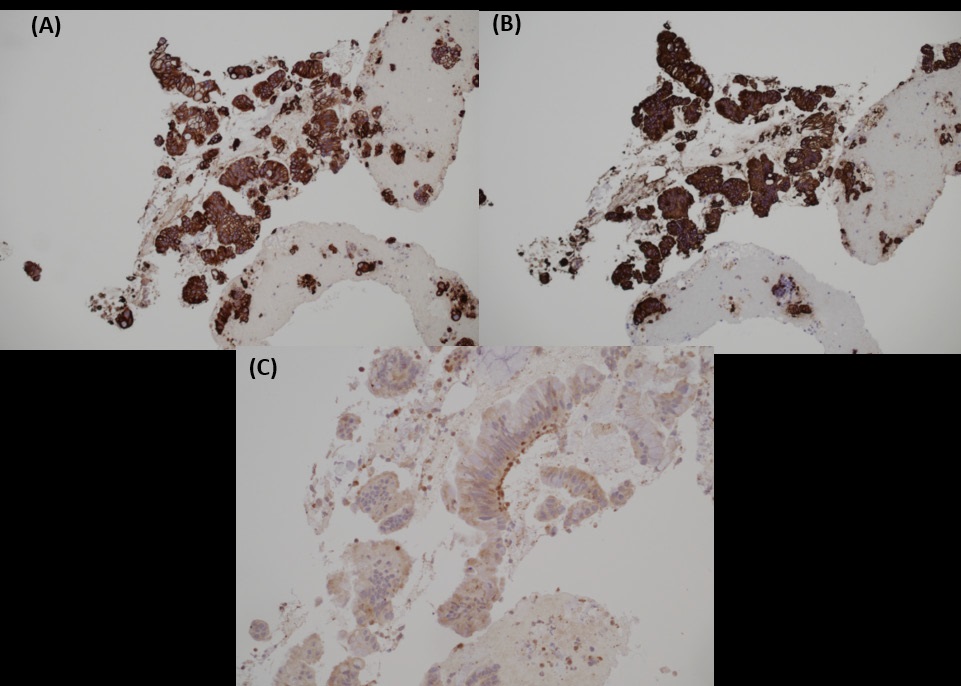
Biopsy from cervix showed infiltration by adenocarcinoma, featuring clusters of malignant glands with angulated contour, glandular fusion and some extravasated mucin in the stroma. The tumour cells contained moderately enlarged, hyperchromatic and pleomorphic nuclei with prominent nucleoli, eosinophilic cytoplasm and readily seen mitoses. The overlying squamous epithelium showed no koilocytosis nor intraepithelial neoplasia (Figure 6). Endometrial aspiration showed detached tumour fragments with similar morphology. No normal endometrial tissue was seen (Figure 7). Immunohistochemically, the tumour cells were negative for HPV surrogate marker (p16) and gastrointestinal markers (CDX-2 and SATB2). Estrogen receptor and progesterone receptor were also negative. SMAD4 immunostain showed loss of nuclear staining (Figure 8). The overall immunoprofile was consistent with pancreatic primary. Biopsies from pancreatic tumour showed blood clot admixed with fragments of tumour cells arranged in strips and fused glands. They contained intracytoplasmic mucin, hyperchromatic pleomorphic nuclei and frequent mitoses (Figure 9). The same panel of immumnostains were repeated, and showed the same results. Additionally, the tumour cells were positive for CK 7 and CK19 (Figure 10).
The tumour in colonic biopsy, cervical biopsy endometrial aspiration and pancreatic biopsy showed similar morphology and same immunostaining results. Hence, the overall features were compatible with pancreatic adenocarcinoma metastasizing to the rectum and cervix.
Discussion
PDAC mostly commonly metastasizes to liver and peritoneum, and metastases to rectum and cervix are extremely rare. In very unusual circumstances, PDAC arising from heterotopic pancreatic tissue along the gastrointestinal tract have been reported, although stomach and the small intestine are more commonly involved [16,17]. Our case demonstrates the potential pitfall of diagnosing adenocarcinoma in colonic and cervical tumour biopsies. In viewing unusual morphology and a lack of precursor lesions, one should alert the possibility of metastasis. PDAC are often well to moderately differentiated, consisting of haphazardly arranged and angulated duct-like glandular structures in densely desmoplastic stroma. Foci of smaller and more irregular glands are usually present as well. Occasionally, more complex glandular formation with multilayered papillary, cribriform, micropapillary or gyriform patterns are noted. Cytologically, the tumour cells are cuboidal to columnar with nuclear pleomorphism and small amount of amphophilic cytoplasm. Precursor lesions, namely pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN), may be seen. Whereas for classical colorectal adenocarcinoma. The typical morphology is architecturally complex, cribriform and fused glands with characteristic Garland’s necrosis in desmoplastic stroma. The tumour cells are more columnar in shape compare to PDAC, with nuclear pseudostratification, vesicular chromatin and prominent nucleoli. Low to high grade dysplasia are often found in the surrounding mucosa. Around 80% of endocervical adenocarcinomas are HPV-associated [18]. The typical morphology is irregular and confluent glands with conspicuous apical mitoses and apoptosis. The neoplastic cells are columnar and mucin-depleted, with elongated pseudostratified nuclei and indistinct cytoplasm. HPV-related lesions such as adenocarcinoma-in-situ, koilocytosis and cervical intraepithelial neoplasia may be seen in adjacent mucosa.
Immunohistochemistry helps to differentiate metastatic PDAC from primary colorectal adenocarcinoma and HPVassociated endocervical adenocarcinoma. CDX2 and SATB2 are sensitive and specific markers for colorectal carcinomas. Immunostain p16 (block-type positivity) and HPV molecular test are useful in diagnosing HPV-associated lesions in the cervix. In the pancreas, no single site-specific marker is available. SMAD4 is a tumour suppressor gene located on chromosome 18q21.1 in the TGF- β signaling pathway [19]. Loss of nuclear SMAD4 expression in immunohistochemistry is a surrogate for SMAD4 gene mutation. It is inactivated in 55% of PDAC due to homozygous deletion or mutation [20]. In contrast, it is mutated in only 10-20% of colorectal carcinomas [21]. No SMAD4 mutation is reported in endocervical adenocarcinoma. Hence, coupled with other appropriate markers, as in our case, SMAD4 is a relatively specific marker for diagnosing PDAC. SMAD4 is yet to be a prognostic marker for PDAC. However, preliminary studies suggestive poor disease-free survival in SMAD4 mutated patients. Last but not least, clinical and radiological correlation are crucial in making the correct diagnosis. As in this case, the concurrent suspicious pancreatic mass in PET scan prompted clinicians and pathologists that metastasis is a possibility.
Conclusion
Metastasis of PDAC to rectum and cervix is exceeding rare. In viewing unusual morphology and a lack of precursor lesions in small biopsies, one should alert the possibility of metastasis. Correlation with clinical history and radiological examination, thorough microscopic examination of tumour morphology, and the use of appropriate immunohistochemistry are essential for making the correct diagnosis. SMAD4 is a relatively specific marker for PDAC. In cases of metastasis of unknown primary, loss of nuclear SMAD4 immunosuppression, coupled with other appropriate markers, is helpful in diagnosing metastatic tumours from pancreas.
References
- Chu LC, Goggins MG, Fishman EK. Diagnosis and Detection of Pancreatic Cancer. Cancer J. 2017 Nov/Dec;23(6):333-342.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69(1): 7-34.
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016; 66(4): 271-89.
- Mayo SC, Nathan H, Cameron JL, Olino K, Edil BH, Herman JM, Hirose K, Schulick RD, Choti MA, Wolfgang CL, Pawlik TM.
- Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012; 118(10): 2674-81.
- Porta M, Fabregat X, Malats N, Guarner L, Carrato A, de Miguel A, Ruiz L, Jariod M, Costafreda S, Coll S, Alguacil J, Corominas JM, Solà R, Salas A, Real FX. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol. 2005; 7(5): 189-97.
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014; 371(11): 1039-49.
- Borad MJ, Saadati H, Lakshmipathy A, et al. Skeletal metastases in pancreatic cancer: a retrospective study and review of the literature. Yale J Biol Med. 2009; 82(1): 1-6.
- Pneumaticos SG, Savidou C, Korres DS, Chatziioannou SN. Pancreatic cancer’s initial presentation: back pain due to osteoblastic bone metastasis. Eur J Cancer Care (Engl). 2010; 19(1): 137-40.
- Bellows C, Gage T, Stark M, McCarty C, Haque S. Metastatic pancreatic carcinoma presenting as colon carcinoma. South Med J. 2009; 102: 748-750.
- Ogu US, Bloch R, Park G. A rare case of metachronous skip metastasis of pancreatic cancer to the colon. Am Surg. 2012; 78: E342-E343.
- Inada K, Shida D, Noda K, Inoue S, Warabi M, Umekita N. Metachronous colonic metastasis from pancreatic cancer seven years post-pancreatoduodenectomy. World J Gastroenterol. 2013; 19(10): 1665-1668.
- BOYSEN TH, McCLOSKEY JF, SCHEFFEY LC. Carcinoma of the pancreas with metastasis to the cervix uteri. Am J Obstet Gynecol. 1951; 61(4): 923-6.
- Kinoshita Y., Yoshizawa K., Yuri T., Tsubur A., Shikata N. Endocervical metastasis of pancreatic cancer: a rare case report of long-term survival. Hum. Pathol. Case Rep. 2016; 5: 34-38
- McCluggage WG, Hurrell DP, Kennedy K. Metastatic carcinomas in the cervix mimicking primary cervical adenocarcinoma and adenocarcinoma in situ: report of a series of cases. Am J Surg Pathol. 2010; 34(5): 735-41.
- Hartsough EM, Erickson BK, Chauhan A, Khalifa MA. Isolated metastatic pancreatic adenocarcinoma to the uterine cervix: A case report. Gynecol Oncol Rep. 2019; 29: 61-63.
- Fukino N, Oida T, Mimatsu K, Kuboi Y, Kida K. Adenocarcinoma arising from heterotopic pancreas at the third portion of the duodenum. World J Gastroenterol. 2015; 21(13): 4082-4088.
- Xiong Y, Xie Y, Jin DD, Wang XY. Heterotopic pancreas adenocarcinoma in the stomach: A case report and literature review. World J Clin Cases. 2020; 8(10): 1979-1987.
- Hodgson A, Olkhov-Mitsel E, Howitt BE, Nucci MR, Parra-Herran C. International Endocervical Adenocarcinoma Criteria and Classification (IECC): correlation with adverse clinicopathological features and patient outcome. J Clin Pathol. 2019; 72(5): 347-353.
- Dardare J, Witz A, Merlin JL, Gilson P, Harlé A. SMAD4 and the TGFβ Pathway in Patients with Pancreatic Ductal Adenocarcinoma. Int J Mol Sci. 2020; 21(10): 3534.
- Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000; 60(7): 2002-2006.
- Miyaki M, Iijima T, Konishi M, et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene. 1999; 18(20): 3098-3103.