Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Short Commentary - Open Access, Volume 3
COVID-19 treatment of pediatric cases: Advantages vs disadvantages
Sedigheh Madani1,2; Sarvenaz Shahin3; Mohammad-Reza Malekpour3; Negar Rezaei1,3*
1 Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran.
2 Department of Pediatrics, Faculty of Medicine, Baqiyatallah University of Medical Sciences, Tehran, IR Iran.
3 Non-Communicable Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran.
*Corresponding Author: Negar Rezaei
Non-Communicable Diseases Research Center,
Endocrinology and Metabolism Population Sciences
Institute, Tehran University of Medical Sciences,
Tehran, Iran.
Email: n.rezaei81@yahoo.com
Received : Jan 24, 2022
Accepted : Feb 24, 2022
Published : Mar 03, 2022
Archived : www.jcimcr.org
Copyright : © Rezaei N (2022).
Citation: Madani S, Shahin S, Malekpour MR, Rezaei N. COVID-19 treatment of pediatric cases: Advantages vs disadvantages. J Clin Images Med Case Rep. 2022; 3(3): 1710.
Short commentary
COVID-19 became the most challenging health, economic, and social problem of the entire world from the beginning of 2020. Despite the first insight about COVID-19 in children, more critical involvement of children was observed. However, World Health Organization has not approved any preventive drug or curative antibiotic for COVID-19 infection [1], and reports on COVID-19 pharmacological treatment in children are limited. Pediatricians have concerns about the effect of common pharmacological treatments of COVID-19 in pediatric cases. Safety and efficacy of pharmacological treatments in hospitalized children are controversial.
Widespread antibiotic regimens were prescribed for hospitalized patients infected by COVID-19 and some suggested treatments of pediatric cases were reported from USA, Italy, and china in previous studies [2-4]. USA national report had mentioned 6% (12/208) pediatric cases received Antiviral and antibacterial drugs [3], and 29% (49/168) children in the multi centric Italian study received hydroxycloroquine, lopinavir/ ritonavir, and/or azithromycin/ clarithromycin [2]. Chinese expert consensus statement revealed that Arbidol is not effective in symptom improvement or shortening of viral shedding in pediatric cases [4]. We wish to present our experience from Iranian COVID-19 registry in the treatment of hospitalized children to illustrate its effect on COVID-19 short time prognosis.
Laboratory confirmed inpatient children 18 yrs were extracted from Iranian COVID-19 registry (February 18th to July 17th, 2020). Individual data were confidential and the study was approved by the Ethical Committee of National Institute for Medical Research Development, NIMAD, Tehran, Iran with approval code (ID: IR.NIMAD.REC.1399.185). Children’s antibiotic therapies relation with Intensive Care Unit (ICU) admission and mortality were analyzed. Administered pharmacological therapies were categorized into anti-viral, anti-bacterial, Azithromycin, and Hydroxychloroquine. Anti-viral treatment consists of all well-known prescribed anti-viral drugs and anti-bacterial treatment consists of all known prescribed anti-bacterial drugs except Azithromycin.
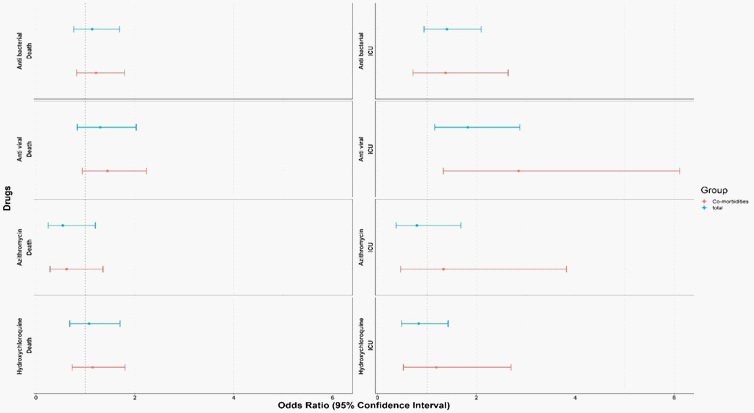
Totally 1,606 hospitalized pediatric cases were included in different age groups; 377 adolescents (12-18 yrs.), 286 children (5-12 yrs.), 165 toddlers (2-5 yrs.), 196 infants 6 months, and 582 neonates and infants 6 months. Two most prescribed therapies for COVID-19 positive hospitalized children were Hydroxychloroquine (14.3%) and Azithromycin (6.9%). Anti-viral and anti-bacterial drugs have been administered in 13.2% and 19.8% children, respectively (Table 1). None of the anti-microbial therapies had significant association with reducing mortality (Figure 1).
Table 1: Treatment types in COVID-19 positive in-patient children (percentages were calculated according to the patients of each age group).
Total |
12-18 Yrs. |
5-12 Yrs. |
2-5 Yrs. |
6M-2 Yrs. |
≤6 M |
Age distribution |
|
319 (20%) |
75 (20%) |
57 (20%) |
44 (26%) |
48 (24%) |
95 (16%) |
Anti-bacterial drug |
All Inpatient |
231 (14%) |
90 (24%) |
34 (12%) |
12 (7%) |
16 (8%) |
79 (13%) |
Hydroxychloroquine |
|
213 (13%) |
70 (18%) |
32 (11%) |
15 (9%) |
21 (11%) |
75 (13%) |
Anti-viral drugs |
|
111 (7%) |
35 (9%) |
22 (8%) |
4 (2%) |
14 (7%) |
36 (6%) |
Azithromycin |
|
84 (30%) |
20 (35%) |
13 (32%) |
9 (31%) |
9 (26%) |
33 (28%) |
Anti-bacterial drugs |
Children With |
50 (18%) |
14 (24%) |
4 (10%) |
3 (10%) |
4 (11%) |
25 (21%) |
Hydroxychloroquine |
|
49 (17%) |
10 (17%) |
6 (14%) |
3 (10%) |
5 (14%) |
25 (21%) |
Anti-viral drugs |
|
26 (9%) |
4 (7%) |
5 (12%) |
0 (0%) |
4 (11%) |
13 (11%) |
Azithromycin |
|
Near half of the Iranian inpatient pediatric cases of COVID-19 have received antibiotics while rate of antibiotic therapies were 26% for Italy and 6% for USA [2,3]. Misuse of antibiotic in human and animals had expedited antibiotic resistance.
To prevent antibiotics’ unwanted side effects in current pandemic, practitioners should consider some points; first, COVID-19 management for inpatients pediatric cases must be according to confirmed laboratory tests of COVID-19, so that the similar symptoms of other viral infections do not confuse them [5,6]. Second, as no anti-viral drug was introduced for COVID-19 [6], pediatricians should prevent inappropriate use of antibiotics. Third, antibiotic therapy is not recommended in COVID-19 pediatric cases because of childhood specific concern about drugs’ side effects [4]. Moreover, simultaneous administration of antibiotics could cause irrecoverable adverse effects in COVID-19 pediatric cases [6]. Although, use of appropriate antibiotic after proof of concomitant co-infection is acceptable, in the absence of sufficient evidence of concomitant, various antibiotics should not prescribed for COVID-19 hospitalized patients.
Misuse of antibiotic in human and animals had expedited antibiotic resistance. Fearless and restive use of various antibiotics in the case of COVID-19 could impose a lot of burden on health system.
While the world is struggling with a critical problem of antibiotic resistance, physicians should administer antibiotics with caution, especially in children, unless clinical trials have revealed the effectiveness of an antiviral or antibacterial drug.
Declarations
Acknowledgements: The authors would like to thank the National Institute for Medical Research Development (NIMAD), Tehran, Iran for their support of this study(grant number: 995531). We also like to thank the Non-Communicable Diseases Research Center’s staff of the Endocrinology and Metabolism Population Sciences Institute of Tehran University of Medical Sciences, Iran Ministry of Health and Medical Education for their wholehearted cooperation.
Ethics: The study was ethically approved by the Ethical Committee of National Institute for Medical Research Development, NIMAD, Tehran, Iran (ID: IR.NIMAD.REC.1399.185).
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Organization WH. Clinical management of COVID-19, interim guidance. WHO: WHO. 2020.
- Garazzino S, et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Eurosurveillance. 2020; 25: 2000600.
- Kim L, et al. Hospitalization rates and characteristics of children aged < 18 years hospitalized with laboratory-confirmed COVID-19—COVID-NET, 14 states, March 1–July 25, 2020. Morbidity and Mortality Weekly Report. 2020; 69: 1081.
- Shen KL, et al. Updated diagnosis, treatment and prevention of COVID-19 in children: Experts’ consensus statement (condensed version of the second edition). World journal of pediatrics: WJP. 2020; 16: 232-239.
- Editorial, Antimicrobial resistance in the age of COVID-19. Nature Microbiology. 2020; 5: 779-779.
- NIH, Potential Antiviral Drugs Under Evaluation for the Treatment of COVID-19, NIH, Editor. 2020.