Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Research Article - Open Access, Volume 3
Increased skin autofluorescence of advanced glycation end-products (AGEs) in Tunisian hemodialysis patients with heart failure
Rim Sakly1; Albert Lecube2; Sabra Aloui3; Khawla Ajimi3; Mouna Hamouda3; Ahmed Letaief3; Fouazi Hawala3; Mariem Ben Salem3; Nouha Ben Mahmoud3; Manel Ben Salah3; Habib Skhiri3; Bruce H R Wolffenbuttel4; Salwa Abid1; Mohsen Kerkeni1*
1 Laboratory for Research on Biologically Compatible Compounds, Faculty of Dental Medicine, University of Monastir, Tunisia.
2 Endocrinology and Nutrition Department, University Hospital Arnau de Vilanova. Obesity, Diabetes and Metabolism (ODIM) Research Group, IRBLleida. University of Lleida, Lleida, Spain.
3 Department of Nephrology, Fattouma Bourguiba University Hospital of Monastir, Tunisia.
4 Department of Endocrinology, University of Groningen, University Medical Center Groningen, The Netherlands.
*Corresponding Author: Mohsen Kerkeni
Higher Institute of Biotechnology Avenue, Tahar
Haddad, BP 745000, Monastir, Tunisia
Email: mohsen.kerkeni@yahoo.fr
Received : Feb 14, 2022
Accepted : Mar 08, 2022
Published : Mar 15, 2022
Archived : www.jcimcr.org
Copyright : © Kerkeni M (2022).
Abstract
Background: Cardiovascular disease (CVD) is the main reason for morbidity and mortality of patients in hemodialyis. Skin autofluorescence (SAF), a noninvasive measurement method, reflects tissue accumulation of advanced glycation end products (AGEs) that has been implicated in CVD as a strong marker. The aim of this study was to evaluate SAF profile in hemodialysis patients and to assess the association between SAF and heart failure.
Methods: In a cross-sectional study, we included 60 hemodialysis (HD) patients who were subdivided in two groups: a HD group without heart failure (n=39) and a HD group with heart failure (n=21). Skin AGEs accumulation was measured by AGE Reader device and clinical data was obtained.
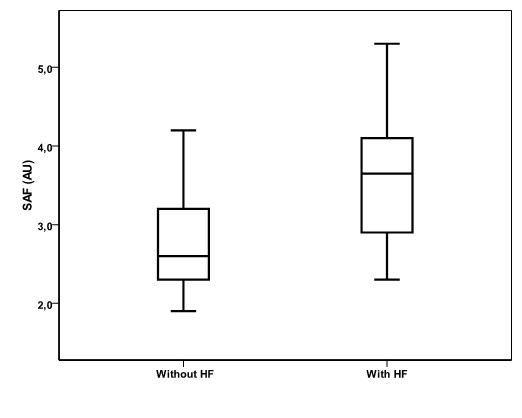
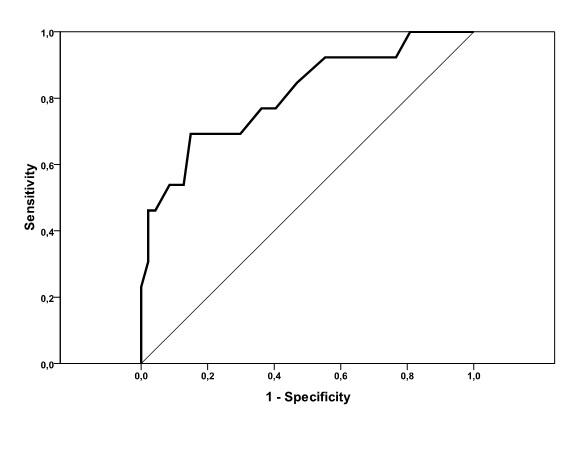
Results: HD patients showed a SAF value at 2.90 (2.40-3.60). HD patients with diabetes mellitus have an increased SAF levels compared to HD patients without diabetes [3.20 (2.90-3.95) vs. 2.70 (2.30-3.30) AU, P = 0.021; respectively]. Furthermore, HD patients with heart failure showed a significant increased SAF levels compared to HD patients without heart failure [3.65 (2.90-4.12) vs. 2.60 (2.30-3.20) AU, P <0.001; respectively]. SAF was associated with age, gender, and duration of dialysis. The ROC analysis indicated that SAF at 3.05 AU was optimal cut-off point for presence of heart failure (P <0.001).
Conclusion: SAF might be a rapid and helpful tool in clinical practice as a potential marker for evaluating and screening heart failure in HD patients non-invasively and might be used as predictor for clinicians.
Keywords: skin autofluorescence; advanced glycation end products; hemodialysis; heart failure.
Abbreviations: AGEs: Advanced glycation end products; SAF: Skin autofluorescence; BMI: Body mass index; CVD: Cardiovascular disease, CKD: Chronic kidney disease, HF: Heart failure.
Citation: Sakly R, Lecube A, Aloui S, Ajimi K, Kerkeni M, et al. Increased skin autofluorescence of advanced glycation endproducts (AGEs) in Tunisian hemodialysis patients with heart failure. J Clin Images Med Case Rep. 2022; 3(3): 1736.
Introduction
Cardiovascular disease (CVD) is the main reason for morbidity and mortality of patients in hemodialyis [1]. During chronic kidney disease (CKD) the metabolic products were accumulated in the body called as uremic toxins. These include advanced glycation end products (AGEs) that are formed non-enzymatically under cumulative glycemic, metabolic and oxidative stress [2,3]. Oxidative stress with uremia increases the inflammatory state and promotes the alterations of proteins, lipids and sugars leading to increase AGEs production [4-6]. Additionally, the impaired excretion of AGEs further contributes to the accumulation in CKD patients and also those in HD. Several studies have shown that the level of plasma or serum Pentosidine, as AGEs products, was increased and related to CKD [7,8] and these observations were also shown previously in Tunisian CKD patients [9].
AGEs accumulate in tissue where they cross-link with proteins such as collagen inducing tissue stiffening of blood vessels and skin. They may also interact with receptor of AGEs (RAGE) which lead to activate intracellular transduction mechanisms resulting in cytokines release, increased oxidative stress and induce tissue damage not only in CKD but also in other diseases. AGEs can be assessed in skin biopsies, but this is an invasive method. The AGE reader, a noninvasive device, is based on the fluorescence properties of AGEs. This method has been validated against specific AGEs levels in skin biopsies in patients with diabetes, hemodialysis and in healthy control subjects [10,11]. Previous studies have shown the association between skin autofluorescence (SAF) and vascular morbidity and mortality in diabetes mellitus [12], hemodialysis [11] and predialysis CKD patients [13,14]. However, data regarding the usefulness of SAF as a predictor of heart failure (HF) in HD patients are limited. Thus, this study aimed firstly to evaluate SAF profile in HD patients with diabetes, hypertension and dyslipidemia, and secondly, to assess the correlation between SAF levels and the presence of heart failure.
Methods
Study population
For the current study, we have included 60 HD patients on March 2021 from Fattouma Bourguiba Hospital at Monastir region (Tunisia), who provided written informed consent. The study protocol was approved by the medical ethical review committee of the hospital. HD patients are subdivided in two groups: HD group without HF (n=39) and HD group with HF (n=21). HF was defined as left ventricular ejection fraction, measured by echocardiography, was below 50%. Diabetes mellitus was defined as fasting blood glucose ≥7.0 mmol/L or HbA1c ≥6.5% and/or use of anti-diabetes medication. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and/or use of anti-hypertensive medication. Dyslipidemia was defined as hypercholesterolemia and/or hypertriglyceridemia and/or use of lipid lowering medication. All HD patients had a standard dialysis (4h, 3times/ week), high flux hemodialysis, polysulfone membrane dialyser and the dialysate solution was free of glucose. The weekly dialysis performance is given as Kt/V (calculated removal of urea by HD).
Clinical data collection
The following data were collected: Age, sex, body mass index (BMI), duration of dialysis, diabetes, hypertension and dyslipidemia. Blood was collected in the fasting state at the morning and transported to the laboratory of biochemistry for biochemical parameters measurement using the standard methods.
Skin autofluorescence measurement
SAF was measured by AGE Reader device (Diagnotics, Groningen, Netherlands) and the method has been described previously [10,15]. The device, a noninvasive tool, evaluates AGEs in skin using the principle that several AGEs emit autofluorescence when excited by UV light. Excitation light source which wavelength is between 300 and 420 nm and the peak intensity is at ~ 370 nm. This light source is projected approximately onto 4 cm2 on the volar side of the forearm skin surface, and the intensity of any light (420-600 nm) emitted is measured with an internal spectrometer. SAF was expressed in arbitrary units (AU) and was calculated from the mean value of the emitted light intensity divided by the excitation light intensity and multiplying by 100. The patients with Fitzpatrick phototypes V and VI were not included due their skin pigmentation, which had ultraviolet reflectance of <6%. The reproducibility of SAF is indicated by a mean coefficient of variance ~5% [15]. SAF was measured three times after dialysis session (taken at different locations on the lower arm with normal skin without scars, tattoos, or other skin abnormalities) and the median value of three SAF measurements were used.
Statistical analysis
Data are shown as mean ± standard deviation (SD) or median and interquartile range in case of non-normally distributed data. Between groups comparisons were performed using the Student’s t-test or Mann-Whitney test, and the correlation coefficient was estimated using the Person or Spearman rank-order correlation analysis. The receiver-operating characteristic (ROC) curves were constructed to determine the optimal SAF cut-off levels to predict the presence of heart failure. A P-value < 0.05 was considered statistically significant. All of statistical analyses were performed using SPSS-17.0 statistical software (IBM, USA).
Results
Characteristics of HD patients
The demographic and clinical characteristics of HD patients are presented in Table 1. HD patients were between 19 and 84 years old, 70% were hypertensive, 38% were dyslipidemic and 21% were diabetic. Dialysis duration was between 9 and 305 months. Mean of Kt/V was 1.64 ± 0.32 and mean of urea reduction rate (URR) was 67.76 ± 7.48. The median SAF value was 2.90 (2.40-3.60). The SAF levels in HD patient with traditional cardiovascular risk factors are presented in Table 2. HD patients with diabetes mellitus has an increased SAF levels compared to HD patients without diabetes [3.20 (2.90-3.95) vs. 2.70 (2.30- 3.30) AU, P = 0.021; respectively]. No differences showed between HD patients with hypertension or with dyslipidemia compared to those who were without hypertension or dyslipidemia but a weak increasing were observed and this may be explained by coexistence of diabetes mellitus.
Table 1: Demographic and clinical data of hemodialysis patients
|
Hemodialysis patients (n = 60) |
Age (years) |
47.57 ± 15.54 |
Male |
36 (60 %) |
BMI (kg/m2) |
24.22 ± 5.23 |
Dialyse duration, (months) |
47 (21-96) |
Kt/V |
1.64 ± 0.32 |
URR (%) |
67.76 ± 7.48 |
Main reasons for dialysis |
|
Primary glomerular Disorders |
14 (23.3%) |
Diabetes nephropathy |
13 (21.6%) |
Renovascular/Hypertensive nephropathy |
13 (21.6%) |
Interstitial nephropathy |
12 (20%) |
Ploycystic nephropathy |
3 (5%) |
Unknown |
5 (8.3%) |
Diabetes mellitus |
13 (21%) |
Hypertension |
42 (70 %) |
Dyslipidemia |
23 (38 %) |
Hemoglobin, g/L |
9.04 ± 1.70 |
Albumin, g/L |
42.58 ± 3.19 |
Fasting glucose, mmol/L |
5.50 (4.60-6.82) |
Triglyceride, mmol/L |
1.50 (1.03-2.39) |
Total cholesterol, mmol/L |
3.85 ± 0.92 |
Skin Autofluorescence, AU |
2.90 (2.40-3.60) |
Data are shown as the mean ± SD, median (interquartile range), or number (percentage). BMI: body mass index
Table 2: SAF levels in HD patients with diabetes mellitus, hypertension and dyslipidemia
HD patients |
SAF (AU) |
P |
Diabetes mellitus (+), (n = 16) |
3.20 (2.90-3.95) |
0.021 |
Diabetes mellitus (-), (n = 44) |
2.70 (2.30-3.30) |
|
Hypertension (+), (n = 42) |
2.95 (2.50-3.65) |
0.297 |
Hypertension (-), (n = 18) |
2.60 (2.27-3.60) |
|
Dyslipidemia (+), (n = 23) |
3.20 (2.70- 3.80) |
0.097 |
Dyslipidemia (-), (n = 37) |
2.70 (2.40-3.20) |
|
Data are shown as the median (interquartile range). (+): With; (-): Without.
Table 3: SAF levels in HD patients with and without heart failure
Hemodialysis patients (n = 60) |
|||
|
H F (-) (n = 39) |
HF (+) (n = 21) |
P |
Age (years) |
46.20 ± 15.26 |
48.44 ± 15.87 |
NS |
Male, n (%) |
27 (64 %) |
9 (50%) |
NS |
BMI (kg/m2) |
23.69 ± 4.84 |
24.97 ± 6.17 |
NS |
Dialysis duration, (months) |
46 (19-81) |
66 (37-127) |
0.045 |
Kt/V |
1.63 ± 0.31 |
1.66 ± 0.32 |
NS |
URR (%) |
66.88 ± 7.58 |
69.72 ± 7.06 |
NS |
Hemoglobin, g/L |
8.80 ± 1.69 |
9.61 ± 1.62 |
NS |
Albumin, g/L |
42.47 ± 3.36 |
42.84 ± 2.82 |
NS |
Fasting glucose, mmol/L |
5.50 (4.60-6.70) |
5.60 (4.65-9.35) |
NS |
Triglyceride, mmol/L |
1.48 (1.00-2.17) |
1.55 (1.10-2.73) |
NS |
Total cholesterol, mmol/L |
3.69 ± 0.88 |
4.11 ± 0.81 |
0.039 |
Skin Autofluorescence, AU |
2.60 (2.30-3.20) |
3.65 (2.90-4.12) |
<0.001 |
Data are shown as the mean ± SD, median (interquartile range), or number (percentage). HF: heart failure BMI: body mass index
SAF levels in HD patients with and without heart failure
SAF levels in HD patients with and without heart failure were shown in table 3. HD patients with HF showed a significant increased SAF levels compared to HD patients without HF [3.65 (2.90-4.12) vs. 2.60 (2.30-3.20) AU, P <0.001; respectively], as shown in figure 1. HD patients with HF showed also a significant increased for dialysis duration and increased total cholesterol compared to HD patients without HF. For correlation of SAF levels with other variables, SAF was associated with age (r = 0.384; P = 0.002), gender (r = - 0.325; P = 0.011) and duration of dialysis (r = 0.285; P = 0.030).
Receiver operating characteristic curve
To predict the presence of HF based on SAF, receiver operating characteristic curve (ROC) was used and shown in figure 2. The maximum Youden index indicated that an SAF at 3.05 AU was optimal cut-off point for the presence of HF. Area under the curve (AUC) = 0.811 (95% CI: 0.699-0.953), P = 0.001 with sensitivity of 77% and specificity of 64%.
Discussion
Several clinical studies have shown a lot of attention about SAF measurement for predicting both microvascular and macrovascular complications in diabetic patients. This cross-sectional study showed that SAF levels were increased in HD patients and the value was markedly increased in HD patients with diabetes or combined with hypertension or dyslipidemia. Furthermore, HD patients with heart failure showed a significant increased SAF compared to HD patients without heart failure. Studies showing the relationship between SAF and CVD in HD patients are limited and reported that SAF is the independent predictor for overall mortality in HD patients [11,16,17]. However several studies have shown the strong association between SAF, CVD and CKD [18-21]. For patients on peritoneal dialysis, one study showed that more deaths were observed in group with high SAF than in group with low SAF group. The SAF values over 3.61 provide a reliable cut-off for predicting three years mortality [22]. The SAF showed to be correlated with the duration of dialysis and glucose exposure dose and independently associated with cardiovascular morbidity [23]. An explanation for the predictive value of SAF could be the main role of AGEs in the development of vascular complications in diabetes mellitus, renal failure and CVD. Besides decreased clearance of AGEs in patients with renal failure, AGEs formation is accelerated throughout the years during dialysis, resulting in progressive AGEs cross-linked to longlived proteins which may contribute to endothelial dysfunction [24,25].
Measurement of SAF can indicate tissue alterations long before they would be revealed by conventional screening methods or cause clinical signs [26]. To shed light, SAF may represent a clinically useful, noninvasively method and fast results for assessing heart failure in HD patients. We observed a significant increased of SAF levels in HD patients with heart failure compared to those without heart failure. Furthermore, to predict the presence of HF in HD patients, we found that the cut-off point of SAF value is was at 3.05 AU. This study demonstrated that SAF >3.05 AU was an important predictor for the presence of HF. Remarkably, this level is the same as used by Wang et al. for predicting abnormal flow-mediated vasodilatation (an indicator of endothelial dysfunction) in uremic subjects on hemodialysis [17]. Previous studies have found an association between SAF and the pathogenesis of HF in diabetic patients without HD. SAF may be a prognostic factor in patients with long-standing persistent atrial fibrillation and the risk value of SAF was considered as 2.6 AU for high CHADS2 score and SAF value at 2.7AU for elevated high sensitivity cardiac troponin levels [27]. Furthermore, SAF can predict the risk of the first HF hospitalization in patients with preserved ejection fraction and the cut-off point of SAF was determined to be > 2.9 AU [28].
In the present study we found significant associations between SAF levels and age, gender and duration of dialysis. Our finding is a line with several studies [11]. McIntyre et al. reported in a cohort of 1707 patients with CKD class III that a large number of cardiovascular and renal risk factors were associated with SAF, such as eGFR, hemoglobin, smoking, total cholesterol, diastolic blood pressure, c-reactive protein, albuminemia, pulse wave velocity, diabetes, and uremic acid [13]. Recently, Shardlow et al. have investigated in a large prospective cohort study that SAF was an independent risk factor for cardiovascular events and all-cause mortality in persons with early CKD [21]. This new finding supports the usefulness of SAF measurement as predictor biomarker for clinicians to detect early disease and monitor patient’s health over time.
Limitations
Some limitations of this study should be noted. This study was conducted at a single center with a relatively small sample size and the value of SAF in predicting for the presence of heart failure could not confirmed in this cross-sectional study, which requires further follow-up.
Conclusion
In conclusion, this study indicates that SAF was associated with the presence of heart failure in Tunisian HD patients. Noninvasive measurement of SAF might be a good surrogate marker for evaluating heart failure. Further investigations in a large number of prospective studies are required to validate the results in this study
Declarations
Acknowledgments: We sincerely thank Professor Bruce H. R. Wolffenbuttel from Department of Endocrinology, University of Groningen, University Medical Center Groningen, the Netherlands for providing the AGE Reader device to our Laboratory research.
Authors’ contributions: MK, Al L and HS: conceived the idea, edited and finalized the manuscript. SA and MK: Performed the study design, skin autofluorescence measurement, data analysis, data interpretation. KA: clinical data collection. Ah L, FH, MBS, NBH and MBS: Helped in the recruitment of patients and clinical fulfillment. All authors read and approved the final manuscript.
Availability of data and materials: The datasets used and/ or analysed during the current study are available from the corresponding author on reasonable request
Consent: The study protocol is in accordance with ethical guidelines of declaration of Helsinki and has approved by the ethics committees at the Hospital of Fattouma Bourguiba at Monastir-Tunisia. All participants signed the informed consent in writing prior to inclusion in the study.
Competing interests: The authors declare that they have no conflict of interest to disclose.
References
- Pozzoni P, Del Vecchio L, Pontoriero G, Filippo S, Locatelli F. Long-term outcome in hemodialysis: morbidity and mortatlity. J Nephrol. 2004; 17: 87-95.
- Arsov S, Graaf R, van Oeveren W, Stegmayr B, Sikole A, Rakhorst G, et al. Advanced glycation end products and skin autofluorescence in end stage renal disease: a review. Clin Chem Lab Med. 2014; 52: 11-20.
- Horner DV, Taal MW. Skin autofuorescence: an emerging biomarker in persons with kidney disease. Curr Opin Nephrol Hypertens. 2019; 28: 507-12.
- Miyata T, Wada Y, Cai Z, et al. Implication of an increased oxidative stress in the formation of advanced glycation end products in patients with end stage renal failure. Kidney Int. 1997; 51: 1170-81.
- Coaccioli S, Standoli ML, Biondi R, et al. Assessment of the oxidative stress markers in patients with chronic renal insufficiency undergoing dialysis treatment. Clin Ter. 2010; 161: 441-44.
- Machowska A, Sun J, Qureshi AR, Isoyama N, Leurs P, Anderstam B, Heimburger O, Barany P, Stenvinkel P, Lindholm B. Plasma Pentosidine and Its Association with Mortality in Patients with Chronic Kidney Disease. PLoS One. 2016; 11: e0163826.
- Furuya R, Kumagai H, Miyata T, Fukasawa H, Isobe S, Kinoshita N, Hishida A. High plasma pentosidine level is accompanied with cardiovascular events in hemodialysis patients. Clin Exp Nephrol. 2012; 16: 421-6.
- Kerkeni M, Saïdi A, Bouzidi H, Letaief A, Ben Yahia S, Hammami M. Pentosidine as a biomarker for microvascular complications in type 2 diabetic patients. Diab Vasc Dis Res. 2013; 10: 239-45.
- Meerwaldt R, Graaff R, Oomen PHN, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gans ROB, Smit AJ. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004; 47: 1324-1330.
- Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, Thorpe SR, Baynes JW, Navis G, Gans RO, Smit AJ. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol. 2005; 16: 3687-93.
- Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, Smit AJ. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care. 2007; 30: 107-12.
- McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Skin autofluorescence and the association with renal and cardiovascular risk factors in chronic kidney disease stage 3. Clin J Am Soc Nephrol. 2011; 6: 2356-63.
- Fraser SD, Roderick PJ, McIntyre NJ, Harris S, McIntyre CW, Fluck RJ, Taal MW. Skin autofluorescence and all-cause mortality in stage 3 CKD. Clin J Am Soc Nephrol. 2014; 9: 1361-8.
- Koetsier M, Lutgers HL, de Jonge C, Links TP, Smit AJ, Graaff R. Reference values of skin autofluorescence. Diabetes Technol Ther. 2010; 12: 399-403.
- Gerrits EG, Lutgers HL, Smeets GH, Groenier KH, Smit AJ, Gans RO, Bilo HJ. Skin autofluorescence: a pronounced marker of mortality in hemodialysis patients. Nephron Extra. 2012; 2: 184- 91.
- Wang CC, Wang YC, Wang GJ, Shen MY, Chang YL, Liou SY, Chen HC, Chang CT. Skin Autofluorescence Is Associated with Endothelial Dysfunction in Uremic Subjects on Hemodialysis. PLoS One. 2016; 11: e0147771.
- Tanaka K, Tani Y, Asai J, Nemoto F, Kusano Y, Suzuki H, Hayashi Y, Asahi K, Katoh T, Miyata T, Watanabe T. Skin autofluorescence is associated with renal function and cardiovascular diseases in pre-dialysis chronic kidney disease patients. Nephrol Dial Transplant. 2011; 26: 214-20.
- Tanaka K, Nakayama M, Kanno M, Kimura H, Watanabe K, Tani Y, Kusano Y, Suzuki H, Hayashi Y, Asahi K, Sato K, Miyata T, Watanabe T. Skin autofluorescence is associated with the progression of chronic kidney disease: a prospective observational study. PLoS One. 2013; 8: e83799.
- Wang AY, Wong CK, Yau YY, Wong S, Chan IH, Lam CW. Skin autofluorescence associates with vascular calcification in chronic kidney disease. Arterioscler Thromb Vasc Biol. 2014; 34: 1784- 90.
- Shardlow A, McIntyre NJ, Kolhe NV, Nellums LB, Fluck RJ, McIntyre CW, Taal MW. The association of skin autofluorescence with cardiovascular events and all-cause mortality in persons with chronic kidney disease stage 3: A prospective cohort study. PLoS Med. 2020; 17: e1003163.
- Mácsai E, Benke A, Kiss I. Skin Autofluorescence and Mortality in Patients on Peritoneal Dialysis. Medicine (Baltimore) 2015; 94: e1933.
- Jiang J, Chen P, Chen J, Yu X, Xie D, Mei C, Xiong F, Shi W, Zhou W, Liu X, Sun S, Zhang P, Yang X, Zhang Y, Zhang Y, Liang X, Zhang Z, Lin Q, Yu Y, Miyata T, Tian J, Liang M, Luo W, Xu X, Hou F. Accumulation of tissue advanced glycation end products correlated with glucose exposure dose and associated with cardiovascular morbidity in patients on peritoneal dialysis. Atherosclerosis. 2012; 224: 187-94.
- Linden E, Cai W, He JC, Xue C, Li Z, Winston J, Vlassara H, Uribarri J. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)- mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin J Am Soc Nephrol. 2008; 3: 691-8.
- Lindsey JB, Cipollone F, Abdullah SM, McGuire DK. Receptor for advanced glycation end-products (RAGE) and soluble RAGE (sRAGE): cardiovascular implications. Diab Vasc Dis Res. 2009; 6: 7-14.
Mácsai E. Skin autofluorescence measurement in the clinical practice of diabetology and nephrology. Orv Hetil. 2012; 153: 1651-7.
Hitsumoto T. Clinical Significance of Skin Autofluorescence in Elderly Patients With Long-Standing Persistent Atrial Fibrillation. Cardiol Res. 2019; 10: 181-87.
Hitsumoto T. Skin Autofluorescence as a Predictor of First Heart Failure Hospitalization in Patients With Heart Failure With Preserved Ejection Fraction. Cardiol Res. 2020; 11: 247-55.