Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 3
Congenital CMV induced neonatal cholestasis: A case report
Reena kumari1*; Richa2; Shashi Sharma3; Lakshay Rana1; Sadadiwala Shikha1; Antan George1
1 Postgraduate Student, Department of Pediatrics, SGT Medical College, Hospital & Research Institute, Budhera, Gurugram, Haryana, India.
2 Assistant Professor, Department of Pediatrics, SGT Medical College, Hospital & Research Institute, Budhera, Gurugram, Haryana, India.
3 Professor, Department of Pediatrics, SGT Medical College, Hospital & Research Institute, Budhera, Gurugram, Haryana.
*Corresponding Author: Reena Kumari
Postgraduate Student, Department of Pediatrics,
SGT Medical College, Hospital & Research Institute, Budhera, Gurugram, Haryana, India.
Email: dr.reenahooda@gmail.com
Received : Jan 24, 2022
Accepted : Mar 17, 2022
Published : Mar 24, 2022
Archived : www.jcimcr.org
Copyright : © Reena Kumari (2022).
Abstract
Cholestatic jaundice in the neonatal period becomes difficult to diagnose because of complex and diverse etiology. The Index case was severe IUGR (intrauterine growth restriction), with a maternal history of infection and leaking per vaginum. The baby cried immediately at birth but was very low birth weight and short for gestation. Soon after birth, the baby developed respiratory distress and had poor activity. The baby was diagnosed of having hyaline membrane disease and early onset of neonatal sepsis and was managed appropriately. Later the baby developed conjugate neonatal hyperbilirubinemia and sepsis was taken as a cause of it and obstructive causes were ruled out. But the baby was not responding well to the treatment and the liver function test remained deranged, so is presented as a diagnostic challenge. The ultrasonography of the abdomen was normal. TORCH (Toxoplasma gondii, Other agents, Rubella, Cytomegalovirus) profile of the baby was sent which turned out to be positive for Cytomegalovirus (CMV). The viral load was high. Hence the baby was treated with Ganciclovir and the baby responded remarkably with the liver enzymes getting reverted to normal and viral load subsequently reduced to zilch. The infant started thriving well and no apparent associated neurological manifestations of the disease were seen in the baby. Currently, the baby is on regular follow-up. The difficulties experienced in the identification of CMV leading to delay in the management of the newborn as well as the positive outcome with Ganciclovir therapy are highlighted in this article. Our goal of reporting this case is to raise pediatricians’ and other stakeholders’ awareness of congenital CMV infection to ensure early detection and appropriate treatment of affected babies, with the ultimate goal of improving their prognosis and preventing the associated audiological and cognitive sequelae.
Keywords: Cholestasis; Cytomegalovirus; Gancyclovir.
Abbreviations: TORCH: Toxoplasma Gondii, Others Agent, Rubella; CMV: Cytomegalovirus; LFT: Liver Function Test; KFT: Kidney Function Test; CBC: Complete Blood Count; IUGR: Intrauterine Growth Restriction; USG: Ultrasonography; CSF: Cerebrospinal Fluid; CRP: C-Reactive Protein; G6PD: Glucose-6 Phosphate Dehydrogenase; HIDA: Hepatobiliary Iminodiacetic Acid; BERA: Bain Stem-Evoked Response Audiometry; PCR: Polymerase Chain Reaction.
Citation: Kumari R, Richa, Sharma S, Rana L, Shikha S, et al. Congenital CMV induced neonatal cholestasis: A case report. J Clin Images Med Case Rep. 2022; 3(3): 1754.
Introduction
Human Cytomegalovirus (CMV) is widespread in the community, and those who become infected stay infected for life, shedding infectious virus intermittently from mucosal surfaces. The human herpesvirus CMV is the largest of the human herpesviruses. Following intrauterine transmission of CMV, congenital CMV infection (present at birth) occurs.
In the United States, rates of congenital infection have been recorded ranging from 0.4 percent to 1.0 percent, with a major multicentre study suggesting a rate of about 0.4 percent. There have also been reports of rates as high as 2% in some parts of Asia and Africa. Following hematogenous transmission of CMV to the placenta, CMV is presumed to be transmitted to the developing fetus by cell-free transfer of virus to the fetal blood system. In women with primary infection during pregnancy, the rate of transmission to the fetus is around 30%; in utero infections also occur in previously immune women (nonprimary infection), albeit at a lower rate, estimated to be on the order of 1–2%. Although the incidence of CMV transmission is higher after primary maternal infection, the absolute number of congenitally infected infants born to women with non-primary infections outnumbers those born to mothers with primary infections by 3-4 times in most communities [1]. This is especially true in Africa, South America, and Asia, where maternal CMV sero-immunity is commonly over 95%. In previously infected women, reinfection with genetically unique strains of CMV occurs, and these newly acquired viruses can be passed to the developing fetus [2]. According to the current study reinfection rates have been reported to be around 15-20 percent in some studies, with yearly rates as high as 25 percent. Immunity to CMV is thus far from protective, even if epidemiological data suggests that it can alter the risk of transmission to the developing fetus. Congenital CMV infection can cause symptomatic illnesses in about 10% of infected babies however 90% of infected children will have no clinical signs of infection during the neonatal period and will only be detected by newborn screening programs [3].
Case report
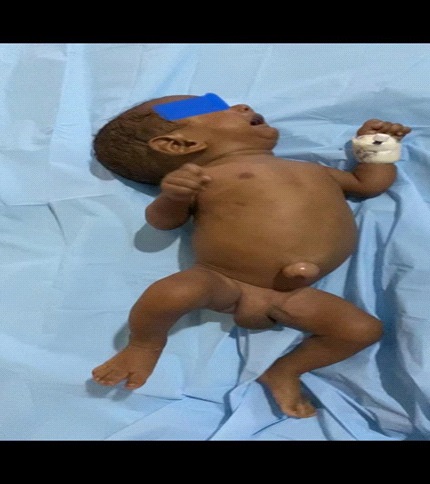
Baby AB was admitted into the special care baby unit of some private hospital of Gurgaon, Haryana shortly after normal vaginal delivery at public sector hospital at a gestational age of 35 weeks due to a history of infection, premature rupture of membrane, and severe IUGR. The baby was born preterm with a birth weight of 1.3 kg, short for gestation, very low birth weight, cried immediately at birth, but soon baby developed respiratory distress, and had poor activity. The baby was diagnosed with hyaline membrane disease and early onset of neonatal sepsis, all these abnormalities were managed appropriately. He was anicteric and had no palpable organ enlargement. complete hemogram revealed thrombocytopenia, (platelet count was 58 x 109), relative lymphocytosis but hematocrit was normal (46.5%) and serum electrolytes were within normal limits. Ultrasonography (USG) skull was normal.
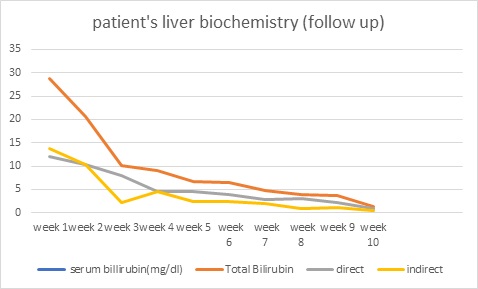
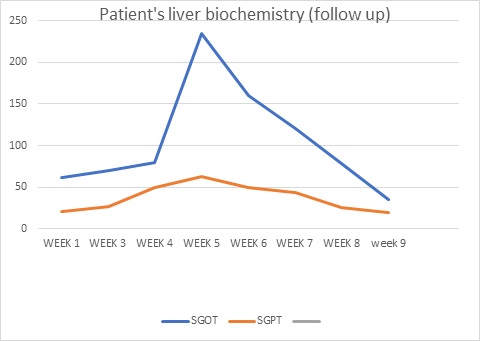
Jaundice appeared on the eighth day of life, then the baby was brought to our hospital with total serum bilirubin of 22.4, direct serum bilirubin 10.8, and indirect serum bilirubin 11.6. CRP (C-Reactive Protein) was raised and CSF (Cerebrospinal Fluid) revealed meningitis. Suspecting biliary obstruction because of sepsis, antibiotics were started, but gradually baby started passing clay-colored stools, developed abdominal distension, which gradually increased causing prominence of superficial veins on the abdomen. Baby’s coagulation profile, was deranged, Liver Function Test (LFT) showed conjugated hyperbilirubinemia with transaminitis, USG cranium, procalcitonin, thyroid profile, G6PD (Glucose-6 Phosphate Dehydrogenase) was normal, blood culture turned out to be sterile, CSF culture showed no growth, direct Coombs test was negative, mother, father, and baby had O positive blood group, so antibiotics planned for 21. Due to financial constraints, the baby was taken to a government centre and was thoroughly investigated. Ultrasound whole abdomen was within normal limits, the urinary reducing substance was negative, HBs Ag/anti-HCV was negative, urine for fungal was negative, LFT, Kidney Function Test (KFT), Complete Blood Count (CBC), coagulation profile was deranged. Antibiotics were upgraded. However gradually he developed ascites and abdominal distension with thrombocytopenia. A liver biopsy was done to determine the cause of cholestasis showed ballooning degeneration of hepatocytes with no evidence of inflammation or bile lakes or extrahepatic bile stasis. The Portal triad was normal. Hence biliary atresia was ruled out. HIDA (Hepatobiliary Iminodiacetic Acid) scan showed hepatomegaly with impaired hepatocellular function and patent bilio-enteric pathway with delayed transit time. The baby continued to deteriorate with deranged liver function tests and was readmitted again to our hospital. We further investigated the infant and the TORCH profile turned out to be positive for Cytomegalovirus. The urine PCR for CMV was also positive and the viral load was 8,000. Hence a diagnosis of congenital Cytomegalovirus infection with cholestasis was kept. Ophthalmology screening for chorioretinitis, BERA (Bain stem-evoked response audiometry) for hearing loss, and neuroimaging to rule out periventricular calcification were done which were normal. With life-threatening symptoms of severe hepatitis and thrombocytopenia patient was started on ganciclovir at 6 mg/kg/dose iv 12 hourly for 6 weeks with CBC, LFT, and KFT monitoring weekly followed by oral valganciclovir at 16 mg/kg/dose for the next 6 months. The LFT as in (Figure 1) came back to normal while no cytomegalovirus was detected on quantitative viral load done on follow-up. The baby started gaining weight and is thriving well.
Discussion
Biochemically, cholestasis is defined as a prolonged increase in conjugated bilirubin levels in the first 14 days of life [4]. Jaundice that develops after 2 weeks, progresses, or does not resolve by this time should be evaluated and a conjugated bilirubin test should be ordered. Infectious, genetic, metabolic, and undefined abnormalities can cause cholestasis in a newborn, obstructing mechanical bile flow or impairing hepatic excretory function and bile secretion. A mechanical lesion of the common bile duct is an obstruction or stricture; the prototypical obstructive condition is biliary atresia. Various congenital defects or damage to the liver or biliary secretory apparatus can impair bile secretion. Neonatal cholestasis is divided into extrahepatic and intrahepatic forms. Any form of cholestasis has similar clinical features. In a neonate with cholestasis, certain entities, such as galactosemia, cystic fibrosis, sepsis, or hypothyroidism can be diagnosed relatively easily as part of most neonatal screening programs. Cholestasis usually has an enigmatic cause.
It’s especially difficult to distinguish between biliary atresia and idiopathic newborn hepatitis following intrauterine infection; CMV is a well-known cause of illness in newborn infants (congenital CMV).
CMV infections can be contracted in a variety of ways, including community exposure, nosocomial transmission, and intrauterine infection. Infants who consume breast milk from seropositive women have a 60 percent chance of being infected [5]. Infants infected by breast milk excrete virus in their saliva and urine for months to years, serving as a reservoir for the spread of the virus to other infants, children, and adults. CMV can be excreted by up to 50% of small infants and children in group care facilities. In addition, infants can pass the virus on to their parents and siblings, allowing CMV to spread throughout the community. Severe and often fatal infections could develop in newborn children lacking antibodies to CMV as a result of being born to mothers without CMV sero immunity or in cases of extreme prematurity. The severe multiorgan disease is uncommon in infants with congenital CMV infections, occurring in less than 5% of cases. Hepatosplenomegaly, petechial rashes, jaundice, and microcephaly are some of the clinical findings in newborns with symptomatic congenital CMV infections [5]. Intrauterine growth restriction has recently been identified as a sign of symptomatic congenital CMV infection by several authors. Direct hyperbilirubinemia, elevated hepatic transaminases, thrombocytopenia, and abnormal cranial ultrasonography/computed tomography findings are all laboratory findings that are consistent with the clinical findings. If the cerebrospinal fluid is obtained, evidence of encephalitis can be found, including an increase in mononuclear cells and, in some cases, an increase in protein. Chorioretinitis will be discovered in a small percentage of symptomatically infected infants (about 10%). Finally, because hearing loss is the most prevalent long-term consequence of congenital CMV infection, an infant’s inability to pass a newborn hearing screening exam should alert caregivers to the possibility of CMV infection [6].
Hearing loss in an older infant or young child should also alert the clinician to the possibility of congenital CMV infection, as approximately half of the infants with hearing loss due to congenital CMV infection will pass an initial hearing screening exam but develop hearing loss later in infancy and early childhood [7]. Long-term monitoring of infected newborns should include an assessment of their growth and neuromuscular function, as well as a referral to specialized treatment if necessary. Hearing loss will develop in roughly 11% of infected infants, and hearing loss will progress in some infants during infancy. In these patients, audiologic tests and follow-up are required. Other complications, such as vision loss, are uncommon, but vision testing and comprehensive eye exams should be part of the treatment plan for newborns with congenital CMV infection. Perinatal infections can occur during birth or as a result of ingesting CMVinfected breast milk. Perinatal infections have nearly never been linked to any clinical symptoms of infection, and perhaps more crucially, no long-term consequences. Perinatal infection can result in severe, widespread infections that can lead to endorgan illness and death in rare situations, such as when CMV is transmitted by breast milk to extremely preterm newborns or infants born to nonimmune women [8]. These more serious infections are thought to arise in infants who lack transplacentally acquired antiviral antibodies, either as a result of extreme preterm or as a result of a mother who lacks anti-CMV antibodies. The recovery of replicating virus and/or viral nucleic acids within the first 2-3 weeks of life is required for the diagnosis of congenital CMV infections. Urine, saliva, and bloods are all sources of the virus and viral nucleic acids. Routine viral culture paired with immunofluorescence and PCR is among the detection methods [9]. Several clinical trials have looked into treating congenitally infected children with ganciclovir, and a considerable number of infected infants have been treated off-label with this medicine due to severe CMV infections [10]. Two trials funded by the NIH’s Collaborative Antiviral Study Group (CASG) suggest that 6 weeks of intravenously administered ganciclovir or 6 months of an oral ganciclovir preparation can help prevent hearing loss and enhance developmental outcomes in infected newborns. Thus, patient education that focuses on describing the sources of infectious virus in communities, as well as general hygiene, could drastically limit CMV dissemination in the community, especially among women of reproductive age.
Conclusion
Though a common cause of neonatal cholestasis, difficulties experienced in the identification of CMV leading to delay in the management of the newborn as well as the positive outcome with Ganciclovir therapy are highlighted in this article. Our goal of reporting this case is to raise pediatricians’ and general physicians’ awareness of congenital CMV infection to ensure early detection and appropriate treatment of affected babies, with the ultimate goal of improving their prognosis and preventing the associated audiological and cognitive sequelae.
Funding: none.
Conflict of interests: None
References
- de Vries JJ, van Zwet EW, Dekker FW, Kroes AC, Verkerk PH, et al. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: A population-based prediction model. Rev Med Virol. 2013; 23: 241-249.
- Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ, et al. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med. 2001; 344: 1366- 1371.
- Swanson EC, Schleiss MR. Congenital cytomegalovirus infection: New prospects for prevention and therapy. Pediatr Clin North Am. 2013; 60: 335-349.
- Feldman AG, Sokol RJ. Neonatal Cholestasis. Neoreviews. 2013; 14: 10.
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: Clinical outcome. Clin Infect Dis. 2013; 57 Suppl 4: S178- 181.
- Fowler KB, Boppana SB. Congenital Cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol. 2006; 35: 226-231.
- Forner G, Abate D, Mengoli C, Palù G, Gussetti N, et al. High Cytomegalovirus (CMV) DNAemia Predicts CMV Sequelae in Asymptomatic Congenitally Infected Newborns Born to Women With Primary Infection During Pregnancy. J Infect Dis. 2015; 212: 67-71.
- Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, et al. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001; 357: 513-518.
- Ross SA, Ahmed A, Palmer AL, Michaels MG, Sánchez PJ, et al. National Institute on Deafness and Other Communication Disorders CHIMES Study. Detection of congenital cytomegalovirus infection by real-time polymerase chain reaction analysis of saliva or urine specimens. J Infect Dis. 2014; 210: 1415-1418.
- Fatema K, Rahman MM, Akhtar S, Shefa J. Efficacy of Valganciclovir versus Ganciclovir in treatment of symptomatic cytomegalovirus infection in infants: An open-label randomized controlled trial. Journal of the International Child Neurology Association. 2019.