Journal of Clinical Images and Medical Case Reports
ISSN 2766-7820
Case Report - Open Access, Volume 3
Unusual cause of dyspnea and hemoptysis in a healthy female
A Bassiri; A Badrinathan; CE Alvarado; CW Towe*
Division of Thoracic and Esophageal Surgery, Department of Surgery, University Hospitals CMC and Case Western Reserve SOM, Cleveland, OH, USA.
*Corresponding Author: Christopher Towe
Division of Thoracic and Esophageal Surgery, Department of Surgery, University Hospitals CMC and Case Western Reserve SOM, Cleveland, OH, USA.
Email: christopher.towe@uhhospitals.org
Received : Feb 23, 2022
Accepted : Mar 18, 2022
Published : Mar 25, 2022
Archived : www.jcimcr.org
Copyright : © Towe C (2022).
Abstract
Rare congenital malformation can lead to the aberrant communication between systemic arterial circulation with pulmonary venous circulation which can range from sequestration to arteriovenous fistulas. We describe a 24 year old female with no significant medical history presenting with dyspnea and hemoptysis. On Computerized Tomography (CT) and angiogram found to have an aberrant vessel branching from celiac axis supplying the right lower lobe and draining into the inferior pulmonary vein with evidence of vascular steal. Due to the size of the vessel, decision was made to forego coil embolization and underwent Video-Assisted Thoracoscopic Surgery (VATS) ligation of the aberrant vessel. She had an uncomplicated operative course and post operatively had resolution of her symptoms. This report demonstrates a rare anatomical congenital vascular malformation causing hemoptysis and resolution of symptoms after ligation of the aberrant blood vessel.
Keywords: pulmonary arteriovenous fistula; vats; hemoptysis.
Citation: Bassiri A, Badrinathan A, Alvarado CE, Towe CE. Unusual cause of dyspnea and hemoptysis in a healthy female. J Clin Images Med Case Rep. 2022; 3(3): 1757.
Introduction
Aberrant systemic arteries draining into the pulmonary venous system are within a spectrum of vascular anomalies ranging from sequestrations to arteriovenous fistulae. The aberrant vessel commonly originates directly from the thoracic aorta. We describe a unique case of the aberrant blood supply originating from the celiac axis and draining into the right inferior pulmonary vein in a healthy young female presenting with dyspnea and hemoptysis.
Case report
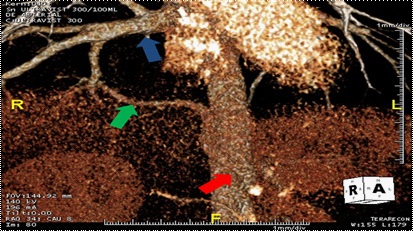
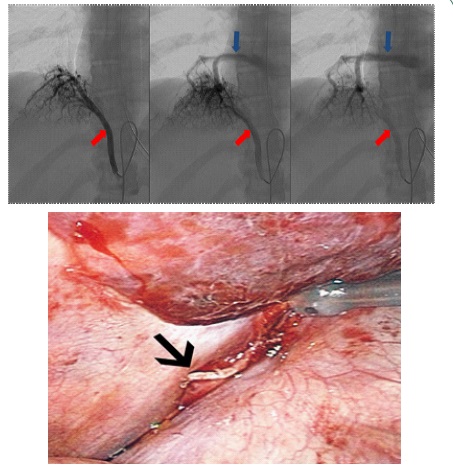
A 24-year-old female with no significant medical history presented with dyspnea. She had no history of chest infections and no abnormalities noted on physical exam. Computerized Tomogram (CT) demonstrated an aberrant vessel originating from the celiac axis, supplying the right lower lobe of the lung and draining into the inferior pulmonary vein. There was no evidence of bronchial sequestration (Figure 1). Intervention was deferred until she presented with worsening dyspnea and hemoptysis. Angiogram delineated a celiac axis branch larger than the splenic and common hepatic arteries with evidence for vascular steal (Figure 2a). Right pulmonary artery angiography demonstrated normal venous return to the left atrium. Given the size of the vessel, coil embolization was deferred and the patient was referred to thoracic surgery. No other anomalies or intracardiac shunts were identified that could explain her symptoms.
Video-Assisted Thoracospic Surgery (VATS) approach was undertaken. Visualization revealed a pulsating and hyperemic right lower lung lobe and an approximately 5 mm vessel entering the right lower lobe at the inferior pulmonary ligament (Figure 2b). Endobronchial and pulmonary surface anatomy were not consistent with sequestration. The vessel was divided with a linear stapler. After ligation of the vessel, intraoperative hemodynamic status remained unchanged. Her postoperative course was uncomplicated. After 2 years of follow-up, the patient reports that she has been able to achieve a level of exercise tolerance in excess of her pre-surgical baseline, and reports no post-operative episodes of hemoptysis.
Discussion
Communications between systemic arteries and pulmonary veins that supply normal lung parenchyma are rare and physiologically distinct from pulmonary sequestration, which is defined by abnormal bronchial anatomy. These vascular anomalies present in two forms: 1) dual blood supply from systemic and pulmonary arteries or 2) a single systemic blood supply to an otherwise normal lung. These communications are typically associated with congenital heart defects.In the case reported, the patient had a systemic and pulmonary arterial supply to a non-sequestered lung.
At 28 days of life, the embryonic lung bud is supplied by multiple primitive arterial vessels that have connections with the dorsal aorta near the celiac axis. By the 6th gestational week, however, the pulmonary arterial system is established, and the splanchnic supply normally regresses. Several factors, such as pulmonary hypoperfusion, may contribute to the persistence of abnormal pulmonary-splanchnic collaterals that supplied the developing lung at inferior levels [1]. These aberrant vessels most commonly originate from the descending aorta and feed the basal pulmonary segments on the left. The affected lung is exposed to systemic pressures, potentially leading to pulmonary hypertension, dyspnea, hemoptysis or high-output cardiac failure; a distinct clinical presentation from sequestration, which typically presents with recurrent pulmonary infections [1]. Symptoms that arise from these abnormal vessels are partly due to anatomic characteristics of the vessel itself; it is a muscular artery when it bifurcates from the aorta and then transitions to an elastic artery when it enters pulmonary parenchyma. As a result, the intrapulmonary portion is exposed to abnormally high (systemic) pressures, and pathologic changes occur that can mimic the pathophysiology of primary pulmonary hypertension [3].
At 28 days of life, the embryonic lung bud is supplied by multiple primitive arterial vessels that have connections with the dorsal aorta near the celiac axis. By the 6th gestational week, however, the pulmonary arterial system is established, and the splanchnic supply normally regresses. Several factors, such as pulmonary hypoperfusion, may contribute to the persistence of abnormal pulmonary-splanchnic collaterals that supplied the developing lung at inferior levels [1]. These aberrant vessels most commonly originate from the descending aorta and feed the basal pulmonary segments on the left. The affected lung is exposed to systemic pressures, potentially leading to pulmonary hypertension, dyspnea, hemoptysis or high-output cardiac failure; a distinct clinical presentation from sequestration, which typically presents with recurrent pulmonary infections [1]. Symptoms that arise from these abnormal vessels are partly due to anatomic characteristics of the vessel itself; it is a muscular artery when it bifurcates from the aorta and then transitions to an elastic artery when it enters pulmonary parenchyma. As a result, the intrapulmonary portion is exposed to abnormally high (systemic) pressures, and pathologic changes occur that can mimic the pathophysiology of primary pulmonary hypertension [3].
Chest CT and contrast enhanced CT angiography are considered the most useful tests to detect the origin and terminus of these vessels. We present a case where the vessel traverses from the celiac axis to the right lower lobe. Cases of a celiac axis origin to the basilar segments are rare and, to our knowledge, have previously been reported as supplying the left lower lobe [2].
Studies show surgical excision and anastomosis of the anomalous artery to pulmonary arteryare successful treatment options for true pulmonary sequestration [4]. In addition, endovascular management for true pulmonary sequestration has been reported in neonates, infants, and adults [5]. Optimal treatment for aberrant arteries without sequestration, however, is unclear. Lobectomy or segmentectomy with division of the anomalous artery has been reported [3]. In this patient, we ligated the vessel without lung resection because the pulmonary arterial supply was normal, and ligation did not result in hypo-perfusion of the lung.
Conclusion
In conclusion, these aberrant vessels, although rare, must be considered in the differential of hemoptysis in an otherwise healthy individual with no previous medical history. Recognition of the existence of these vascular anomalies, their common pathways and distributions will prevent iatrogenicinjury of such vessels during thoracic and abdominal cavity dissection.
References
- Yamanaka A, Hirai T, Fujimoto T, Hase M, Noguchi M, Konishi F, et al. Anomalous systemic arterial supply to normal basal segments of the left lower lobe. Ann Thorac Surg. 1999; 68: 332–338.
- Irodi A, Cherian R, Keshava SN, James P. Dual arterial supply to normal lung: Within the sequestration spectrum. Br J Radiol. 2010; 83: e86–e89
- Mori S, Odaka M, Asano H, Marushima H, Yamashita M, Kamiya N, et al. Anomalous systemic arterial supply to the basal segments of the lung: Feasible thoracoscopic surgery. Ann Thorac Surg. 2013; 96: 990-994
- Zhang SX, Wang HD, Yang K, Cheng W, Wu W, et al. Retrospective review of the diagnosis and treatment of pulmonary sequestration in 28 patients: surgery or endovascular techniques? J Thorac Dis. 2017; 9: 5153-5160.
- Marine L, Valdes F, Mertens V, Bergoeing M, Kramer A, et al. Endovascular treatment of symptomatic pulmonary sequestration. Ann Vasc Surg. 2011; 25: 696e11-699e15.